Cyclophane: Difference between revisions
m robot Adding: fr:Cyclophane |
m +ja |
||
| Line 69: | Line 69: | ||
[[fr:Cyclophane]] |
[[fr:Cyclophane]] |
||
[[it:Ciclofani]] |
[[it:Ciclofani]] |
||
[[ja:シクロファン]] |
|||
[[pl:Cyklofany]] |
[[pl:Cyklofany]] |
||
[[zh:环芬]] |
[[zh:环芬]] |
||
Revision as of 15:28, 4 September 2009
A cyclophane is a hydrocarbon consisting of an aromatic unit (typically a benzene ring) and an aliphatic chain that forms a bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cagelike structures are also known. Cyclophanes are well-studied in organic chemistry because they adopt unusual chemical conformations due to build-up of strain. Despite this, cyclophane structures are not unknown to biomolecules.
![Scheme 2. [6]paracyclophanes](/upwiki/wikipedia/commons/thumb/d/d9/-6-cyclophanes.png/300px--6-cyclophanes.png)
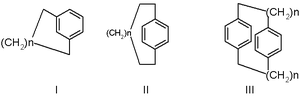
Basic cyclophane types are [n]metacyclophanes (I) in scheme 1, [n]paracyclophanes (II) and [n,n']cyclophanes (III). the prefixes meta and para correspond to the usual arene substitution patterns and n refers to the number of atoms making up the bridge.
Structure
Paracyclophanes adopt the boat conformation normally observed in cyclohexanes but are still able to retain aromaticity. The smaller the value of n the larger the deviation from aromatic planarity. In [6]paracyclophane which one of the stable cyclophanes X-ray crystallography shows that the aromatic bridgehead carbon atom makes an angle of 20.5° with the plane. The benzyl carbons deviate by another 20.2°. The carbon to carbon bond length alternation has increased from 0 for benzene to 39 pm.[1][2]
In organic reactions [6]cyclophane tends to react as a diene derivative and not as an aromat. With bromine it gives 1,4-addition and with chlorine the 1,2-addition product forms.
Yet the proton NMR spectrum displays the aromatic protons and their usual deshielded positions around 7.2 ppm and the central methylene protons in the aliphatic bridge are even severely deshielded to a position of around - 0.5 ppm, that is, even deshielded compared to the internal reference tetramethylsilane. With respect to the diamagnetic ring current criterion for aromaticity this cyclophane is still aromatic.
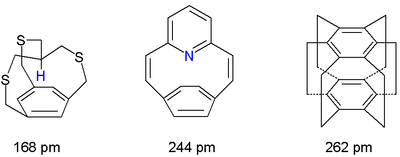
One particular research field in cyclophanes involves probing just how close atoms can get above the center of an aromatic nucleus.[3] In so-called in-cyclophanes with part of the molecule forced to point inwards one of the closest hydrogen to arene distances experimentally determined is just 168 picometer.
A non-bonding nitrogen to arene distance of 244 pm is recorded for a pyridinophane and in the totally weird superphane the two benzene rings are separated by a mere 262 pm. Another representative of this group are in-methylcyclophanes.
Synthetic methods
[6]paracyclophane can be synthesized[4][5] in the laboratory by a Bamford-Stevens reaction with spiro ketone 1 in scheme 3 rearranging in a pyrolysis reaction through the carbene intermediate 4. The cyclophane can be photochemically converted to the Dewar benzene 6 and back again by application of heat. A separate route to the Dewar form is by a cationic silver perchlorate induced rearrangement reaction of the bicyclopropenyl compound 7.
![Scheme 3. [6]paracyclophane synthesis](/upwiki/wikipedia/commons/thumb/3/31/-6-cyclophaneSynthesis.png/500px--6-cyclophaneSynthesis.png)
Metaparacyclophanes constitute another class of cyclophans like the [14][14]metaparacyclophane[6] in scheme 4[7] featuring a in-situ Ramberg-Bäcklund Reaction converting the sulfone 3 to the alkene 4.
![Scheme 4. [14][14]metaparacyclophane](/upwiki/wikipedia/commons/thumb/9/93/Metaparacyclophane.png/600px-Metaparacyclophane.png)
Naturally occurring cyclophanes
Despite carrying strain, the cyclophane motif does exist in nature. One example of a metacyclophane is cavicularin.
Haouamine A is a paracyclophane found in a certain species of tunicate. Because of its potential application as an anticancer drug it is also available from total synthesis via an alkyne - pyrone Diels-Alder reaction in the crucial step with expulsion of carbon dioxide (scheme 5).[8]
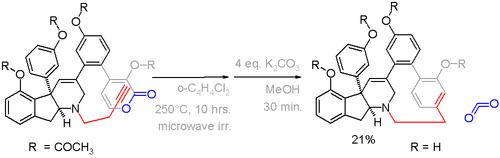
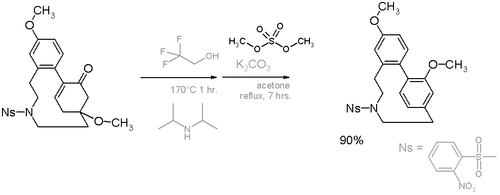
In this compound the deviation from planarity is 13° for the benzene ring and 17° for the bridgehead carbons.[9] An alternative cyclophane formation strategy in scheme 6[10] was developed based on aromatization of the ring well after the formation of the bridge.
[n,n]Paracyclophanes
A well exploited member of the [n,n]paracyclophane family is [2,2]paracyclophane. One method for its preparation is by a 1,6-Hofmann elimination:[11]
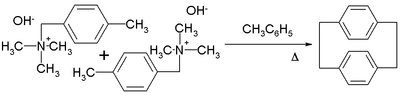
The [2.2]paracyclophane-1,9-diene has been applied in ROMP to a poly(p-phenylene vinylene) with alternating cis-alkene and trans-alkene bonds using Grubbs' second generation catalyst:[12]
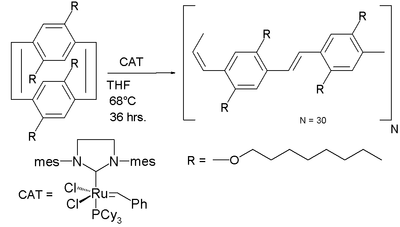
The driving force for ring-opening and polymerization is strain relief. The reaction is believed to be a living polymerization due to the lack of competing reactions.
Because the two benzene rings are in close proximity this cyclophane type also serves as guinea pig for photochemical dimerization reactions as illustrated by this example:[13]
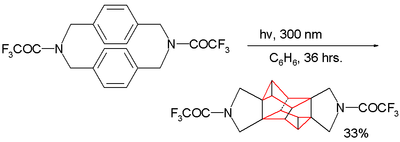
The product formed has an octahedrane skeleton. Interestingly when the amine group is replaced by a methylene group no reaction takes place: the dimerization requires through-bond overlap between the aromatic pi electrons and the sigma electrons in the C-N bond in the reactants LUMO.
References
- ^ Synthesis and molecular structure of (Z)-[6]Paracycloph-3-enes Yoshito Tobe, Kenichi Ueda, Teruhisa Kaneda, Kiyomi Kakiuchi, Yoshinobu Odaira, Yasushi Kai, Nobutami Kasai J. Am. Chem. Soc.; 1987; 109(4); 1136-1144. Abstract
- ^ J. Hunger, C. Wolff, W. Tochtermann, E-M. Peters, K.Peters, H.G. von Schering Chem. Ber., 119, 2698 (1986)
- ^ Molecular Iron Maidens: Ultrashort Nonbonded Contacts in Cyclophanes and Other Crowded Molecules Robert A. Pascal, Jr Eur. J. Org. Chem. 2004, 3763-3771 doi:10.1002/ejoc.200400183
- ^ [6]Paracyclophane Vinayak V. Kane, Anthony D. Wolf, Maitland Jones, , Jr. J. Am. Chem. Soc.; 1974; 96(8); 2643-2644. Abstract
- ^ Interconversion of [6]paracyclophane and 1,4-hexamethylene(Dewar benzene) Seetha L. Kammula, Linda D. Iroff, Maitland Jones, , Jr. J. W. Van Straten, W. H. De Wolf, F. Bickelhaupt J. Am. Chem. Soc.; 1977; 99(17); 5815-5815. Abstract
- ^ [14][14]Metaparacyclophane: First Example of an [m][n]MetaparacyclophaneChunmei Wei, Kai-For Mo, and Tze-Lock Chan J. Org. Chem.; 2003; 68(7) pp 2948 - 2951; (Note) Abstract
- ^ Scheme 4. Reaction scheme: with para-ring in place ring closure of meta part by nucleophilic displacement of dibromide by disulfide. Then oxidation of sulfide to sulfone by hydrogen peroxide followed by in-situ Ramberg-Bäcklund Reaction with halide donor dibromodifluoromethane and base potassium hydroxide. Final step hydrogenation pf alkene by hydrogen and palladium on carbon
- ^ Total Synthesis of (±)-Haouamine A Phil S. Baran and Noah Z. Burns J. Am. Chem. Soc.; 2006; ASAP Web Release Date: 04-Mar-2006; Abstract The authors mark the biosynthetic origin as mysterious
- ^ Synthesis of the 3-Aza-[7]-paracyclophane Core of Haouamine A and B Peter Wipf and Markus Furegati Org. Lett.; 2006; 8(9) pp 1901 - 1904; (Letter) Abstract
- ^ Scheme 6. Reaction scheme: step I elimination reaction of methanol with trifluoroethanol and diisopropylamine, step II methylation with dimethyl sulfate. Ns = Nosylate
- ^ Organic Syntheses, Coll. Vol. 5, p.883 (1973); Vol. 42, p.83 (1962) Link.
- ^ Soluble Poly(p-phenylenevinylene)s through Ring-Opening Metathesis Polymerization Chin-Yang Yu and Michael L. Turner Angew. Chem. Int. Ed. 2006, 45, 7797 –7800 doi:10.1002/anie.200602863
- ^ Photoreaction of a 2,11-Diaza[3.3]paracyclophane Derivative: Formation of Octahedrane by Photochemical Dimerization of Benzene Hideki Okamoto, Kyosuke Satake, Hiroyuki Ishida, and Masaru Kimura J. Am. Chem. Soc.; 2006; 128(51) pp 16508 - 16509; (Communication) doi:10.1021/ja067350r
