Carbon suboxide: Difference between revisions
Additional info to preview |
What does evil smell like? |
||
| Line 61: | Line 61: | ||
while [[Otto Diels]] later stated that the more organic names dicarbonyl methane and dioxallene were also correct. |
while [[Otto Diels]] later stated that the more organic names dicarbonyl methane and dioxallene were also correct. |
||
It is most commonly described as an evil-smelling gas. |
It is most commonly described as an evil-smelling gas.{{fact}} |
||
==Synthesis== |
==Synthesis== |
||
Revision as of 16:26, 5 November 2009
| |

| |
| Names | |
|---|---|
| IUPAC name
propa-1,2-diene-1,3-dione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3O2 | |
| Molar mass | 68.0309 g mol−1 |
| Appearance | colorless gas strong, pungent odor |
| Density | 0.906 ± 0.06 g/cm3, gas at 298 K |
| Melting point | −111.3 °C |
| Boiling point | 6.8 °C |
Refractive index (nD)
|
1.4538 (0 °C) |
| Structure | |
| linear | |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
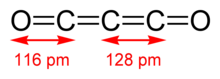
Carbon suboxide, or tricarbon dioxide, is an oxide of carbon with chemical formula C3O2 or O=C=C=C=O. Its four cumulative double bonds make it a cumulene. It is one of the stable members of the series of linear oxocarbons O=Cn=O, which also includes carbon dioxide (CO2) and pentacarbon dioxide (C5O2).
Brodie discovered it in 1873 by submitting electric current to carbon monoxide.[1][2] Marcellin Berthelot created the name carbon suboxide, [3] while Otto Diels later stated that the more organic names dicarbonyl methane and dioxallene were also correct.
It is most commonly described as an evil-smelling gas.[citation needed]
Synthesis
It is synthesized by warming a dry mixture of phosphorus pentoxide (P4O10) and malonic acid or the esters of malonic acid.[4] Therefore, it can be also considered as the anhydride of malonic anhydride, i.e. the "second anhydride" of malonic acid. Malonic anhydride (not to be confused with maleic anhydride) is a real molecule.[5]
Several other ways for synthesis and reactions of carbon suboxide can be found in a review from 1930 by Reyerson.[6]
Carbon suboxide polymerizes spontaneously to a red, yellow, or black solid. The structure is postulated to be poly(α-pyronic), similar to the structure in 2-Pyrone (α-Pyrone). [7][8] In 1969, it was hypothesized that the color of Martian surface was caused by this compound; this was disproved by the Viking Mars probes.[9]
Uses
Carbon suboxide is used in the preparation of malonates; and as an auxiliary to improve the dye affinity of furs.
See also
- Carbon subsulfide, C3S2, whose structure is S=C=C=C=S
- Carbon monoxide (CO)
- Dicarbon monoxide (C2O)
- Dicarbon dioxide (C2O2)
References
- ^ Brodie B. C. (1873). "Note on the Synthesis of Marsh-Gas and Formic Acid, and on the Electric Decomposition of Carbonic Oxide". Proceedings of the Royal Society (London). 21: 245–247. doi:10.1098/rspl.1872.0052.
- ^ Brodie B. C. (1873). "Über eine Synthese von Sumpfgas und Ameisensäure und die electrische Zersetzung des Kohlenoxyds". Annalen der Chemie. 169: 270. doi:10.1002/jlac.18731690119.
- ^ Marcellin Berthelot (1891). "Action de la chaleur sur l'oxyde de carbone". Annales de chimie et de physique. 6 (24): 126–132.
- ^ Diels O, Wolf B (1906). "Ueber das Kohlensuboxyd. I". Chemische Berichte. 39: 689–697. doi:10.1002/cber.190603901103.
- ^ SpringerLink - Journal Article
- ^ Reyerson L. H., Kobe K. (1930). "Carbon Suboxide". Chemical Reviews. 7: 479–492. doi:10.1021/cr60028a002.
- ^
M. Ballauff, L. Li, S. Rosenfeldt, N. Dingenouts, J. Beck, P. Krieger-Beck (2004). "Analysis of Poly(carbon suboxide) by Small-Angle X-ray Scattering". Angewandte Chemie International Edition. 116 (43): 5843–5846. doi:10.1002/anie.200460263.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ A. Ellern, T. Drews, K. Seppelt (2001). "The Structure of Carbon Suboxide, C3O2, in the Solid State". Zeitschrift für anorganische und allgemeine Chemie. 627 (1): 73–76. doi:10.1002/1521-3749(200101)627:1<73::AID-ZAAC73>3.0.CO;2-A.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ William T. Plummer & Robert K. Carsont (1969). "Mars: Is the Surface Colored by Carbon Suboxide?". Science. 166: 1141. doi:10.1126/science.166.3909.1141.
External links
