Wikipedia:Reference desk/Science: Difference between revisions
| Line 657: | Line 657: | ||
Somehow I'm thinking that at that wavelength something mysterious happens like the propagation in a single direction stops and you get a stationary oscillation that is self containing otherwise known as energy in the form of mass. Sort of like a cowboy rope trick where the rope spins in a loop and then the loop moves forward and backward or some such crazy back and forth but stationary relationship like that. <small>[[Special:Contributions/71.100.0.254|71.100.0.254]] ([[User talk:71.100.0.254|talk]]) 03:15, 8 November 2009 (UTC)</small> |
Somehow I'm thinking that at that wavelength something mysterious happens like the propagation in a single direction stops and you get a stationary oscillation that is self containing otherwise known as energy in the form of mass. Sort of like a cowboy rope trick where the rope spins in a loop and then the loop moves forward and backward or some such crazy back and forth but stationary relationship like that. <small>[[Special:Contributions/71.100.0.254|71.100.0.254]] ([[User talk:71.100.0.254|talk]]) 03:15, 8 November 2009 (UTC)</small> |
||
= November 8 = |
|||
== Convict Fish == |
== Convict Fish == |
||
Revision as of 05:50, 8 November 2009
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
November 2
Hammerhead Sharks
In what part of the world are hammerhead sharks usually found? The article on them didn't give any specific locations.99.251.239.89 (talk) 00:25, 2 November 2009 (UTC)
- Hammerhead is a genus name and is pretty broad. Click on the individual species links (e.g. Scalloped hammerhead, Great hammerhead) and you'll see little maps. --Mr.98 (talk) 00:45, 2 November 2009 (UTC)
- (edit conflict) That would depend on which shark you wanted. ~ Amory (u • t • c) 00:48, 2 November 2009 (UTC)
- Cute. DRosenbach (Talk | Contribs) 15:52, 2 November 2009 (UTC)
h1n1 vaccine
hey is there blood in the h1n1 vaccine? i cant find any info on whats in it.--Least0190 (talk) 00:28, 2 November 2009 (UTC)
- I don't think there is blood (human or otherwise) in any modern vaccine. --Mr.98 (talk) 00:35, 2 November 2009 (UTC)
- However, there are chicken egg proteins in it, so if you have any allergies to chicken eggs, you may not be able to take that vaccine. See also 2009 flu pandemic vaccine for the H1N1 (swine flu) vaccine, and Influenza vaccine for flu vaccines in general. --Jayron32 00:40, 2 November 2009 (UTC)
- (edit conflict) There most definitely is not. The only people who are injected with any sort of blood are heroin
eaddicts. ~ Amory (u • t • c) 00:40, 2 November 2009 (UTC)- For proof (sort of) you can read Hemagglutinin (influenza). Essentially, flu surface proteins cause blood to clump in vitro, which would make for a very ineffective vaccine. ~ Amory (u • t • c) 00:43, 2 November 2009 (UTC)
- {Sidetrack: While I am personally addicted to heroes, I am doubtful about Amory's claim above that heroin addicts are "injected with blood". There may be blood in dirty needles, but there is, as far as I know, no intent to inject blood. ( Blood dopers may inject blood, but that has nothing to do with heroin.) Is there some treatment technique that involves blood injections for addicts?} Bielle (talk) 00:56, 2 November 2009 (UTC)
- Not a treatment by any means - Heroin users who want the hit to hit faster and the high to be higher have been known to stick the needle in their arm, draw a little bit of blood up into the syringe, then inject the mixture back in. It's a great way to OD or get bubbles into your bloodstream, and a really, really, really stupid thing to do, but then so is doing heroin. Trainspotting features this technique, iirc. ~ Amory (u • t • c) 01:00, 2 November 2009 (UTC)
- I am now better educated. Thank you, Amory. Bielle (talk) 01:04, 2 November 2009 (UTC)
- Honestly this doesn't make sense to me. How would this get the heroin to your brain any faster? I had a vague notion that the idea was precisely to prevent bubbles — once your blood was up in the syringe, you knew there was no air in the needle. I don't know whether that makes sense either, but it's more plausible than that it makes you high faster. --Trovatore (talk) 01:43, 2 November 2009 (UTC)
- Likely there are 2 good reasons for drawing a little blood, 1. you are sure that you are actually in a vein. 2. you ensure that there isn't too much heroin wasted residually in the needle. Unomi (talk) 01:53, 2 November 2009 (UTC)
- Ah right, the vein thing, I think that was it, not the bubbles. --Trovatore (talk) 04:00, 2 November 2009 (UTC)
- Well, for one, some people think that mixing powder with water weakens it, so they just put the powder in the syringe, then use the blood to put it in solution. ~ Amory (u • t • c) 06:20, 2 November 2009 (UTC)
- Ah right, the vein thing, I think that was it, not the bubbles. --Trovatore (talk) 04:00, 2 November 2009 (UTC)
- Likely there are 2 good reasons for drawing a little blood, 1. you are sure that you are actually in a vein. 2. you ensure that there isn't too much heroin wasted residually in the needle. Unomi (talk) 01:53, 2 November 2009 (UTC)
- Honestly this doesn't make sense to me. How would this get the heroin to your brain any faster? I had a vague notion that the idea was precisely to prevent bubbles — once your blood was up in the syringe, you knew there was no air in the needle. I don't know whether that makes sense either, but it's more plausible than that it makes you high faster. --Trovatore (talk) 01:43, 2 November 2009 (UTC)
- I am now better educated. Thank you, Amory. Bielle (talk) 01:04, 2 November 2009 (UTC)
- Not a treatment by any means - Heroin users who want the hit to hit faster and the high to be higher have been known to stick the needle in their arm, draw a little bit of blood up into the syringe, then inject the mixture back in. It's a great way to OD or get bubbles into your bloodstream, and a really, really, really stupid thing to do, but then so is doing heroin. Trainspotting features this technique, iirc. ~ Amory (u • t • c) 01:00, 2 November 2009 (UTC)
- Well, there's one pretty blatant error above -- people who get a blood transfusion are certainly injected with blood, obviously. Looie496 (talk) 02:42, 2 November 2009 (UTC)
- I left an obnoxious semantic loophole there for myself - transfusions aren't so much injected, per se, as they are dripped. ~ Amory (u • t • c) 06:13, 2 November 2009 (UTC)
ok thanks guys its just that i read that it has pig and horse blood in it, i dont want animals blood in me.--Least0190 (talk) 03:27, 2 November 2009 (UTC)
- If you want it, here's a link. You can read through the full contents of each vaccine, and see that none of them contain anything like that. The closest you get, as Jayron said, is the possibility of less than one millionth of a gram of egg proteins. ~ Amory (u • t • c) 06:20, 2 November 2009 (UTC)
- May I suggest for you Carl Sagan's The Demon-Haunted World? Imagine Reason (talk) 19:13, 2 November 2009 (UTC)
Is it possible for energy/ information to travel faster than speed of light in the following case
Let we have a straight pipe AB long but greater than 300,000 km in the free space or vacuum. The pipe is full of small balls of perfect shapes from point A to B and each ball is connected to each other just like two circles at their common tangent point. Assume there is no friction between the pipe and balls. For simplicity we can also ignore the pipe.
Would the last ball at point B be move out if we add a ball to point A of the pipe in less than a second, if not why?
I know nothing can travel greater than speed of light but I'm just asking if it might possible. Thanks 68.147.38.24 (talk) 01:34, 2 November 2009 (UTC) khattak
- Nothing can be perfectly rigid. The balls are made up of atoms. When you push on the first ball, what happens? Its atoms get pushed closer together. They don't want to be closer together, so they start pushing on other atoms behind them, which move farther away. This generates a compression wave down the pipe, which moves quite fast, but slower than light. (It probably moves at about the speed of sound in whatever the balls are made of.) --Trovatore (talk) 01:38, 2 November 2009 (UTC)
Thank you for your swift reply but sorry to ask you again. Would your answer be the same if two persons commune each other by pushing the same whole pipe or rod in dark space against one another? Because there is diference between the pushing speed of atoms and the pushing speed of the whole pipe/ rod 68.147.38.24 (talk) 03:42, 2 November 2009 (UTC)khattak
- "The whole pipe/rod" is an object composed of atoms. It is no more solid and no more rigid than its full packing of balls. DMacks (talk) 03:48, 2 November 2009 (UTC)
- Trovatore and DMacks are correct. See "Faster-than-light#FTL_phenomena" for some interesting findings. Axl ¤ [Talk] 07:10, 2 November 2009 (UTC)
Supposing the rod was made of quark matter?80.2.195.180 (talk) 12:30, 2 November 2009 (UTC)Trevor Loughlin
- Ho hum, I asked this question a while back - revisiting the archive, I see I forgot to respond, so here's a belated thanks to everyone who answered me. Here is that discussion. Vimescarrot (talk) 13:38, 2 November 2009 (UTC)
- This question is asked so often that it has made me wonder if we have a template for the constant answer that keeps getting rehashed over and over. -- kainaw™ 15:09, 2 November 2009 (UTC)
- Hmmm... I just thought that if I have time today, I will trek through the archives and get a list of links to the previous questions. There should be at least 100 of them. Then I can make a template that says: "Your question has been asked and answered here and here and here and here and here and here and here...." -- kainaw™ 15:10, 2 November 2009 (UTC)
- Hmm... seems like more work than just answering it again, in most cases (or, putting it another way—it is probably easier for four people to recapitulate parts of an answer than it is for one person to look up all of the previous answers. Also more fun, for the four people). This one is common enough in general that we could have just provided Google links—[1]. --Mr.98 (talk) 16:10, 2 November 2009 (UTC)
- That information cannot travel faster than the speed of light (locally, at least) is a fundamental principle of special and general relativity. Those theories have been very successful at making predictions we have experimentally verified, so we assume that principle is correct. That means that there is a fundamental limit on the rigidity of any object, whatever its composition, since it the speed of sound in it (which is what we are talking about, really) must be slower than the speed of light. --Tango (talk) 18:34, 2 November 2009 (UTC)
- Short answer: in order to change what is happening, you need to apply a force. Either you force something directly, or apply a force to something which applies a force to something else ... We only have four forces - electromagnetism, gravity, strong, and weak, and all four are transmitted (over long distances) at the speed of light. No force travels faster than light, so your effect can't be transmitted faster than light. -- 128.104.112.149 (talk) 22:15, 2 November 2009 (UTC)
- What about Quantum entanglement? From the dictionary: a quantum mechanical phenomenon in which the quantum states of two or more objects have at all times to be described with reference to each other, each instantaneously tracking changes to the other, however large the spatial separation of the objects. Instantaneously, no matter what the distance. That's faster than the speed of light. 20.137.18.50 (talk) 17:51, 3 November 2009 (UTC)
- It's only in a very limited way that quantum entanglement can violate locality. Regardless of quantum entanglement, information can't travel faster than the speed of light, which applies in the case of pushing a long rod. See no-communication theorem. Red Act (talk) 18:42, 3 November 2009 (UTC)
- In short - the quantum states being transmitted are random, so you can't use them to communicate anything other than random values. I did see a proposal that they could be used to communicate a random key for encryption. The encrypted information would then be transmitted by conventional means. --Tango (talk) 18:47, 3 November 2009 (UTC)
- I got rid of the wrong definition at Wiktionary. The original entry, created on August 10, 2005, was okay, if not very edifying. Five days later it was replaced with the incorrect one, which survived for more than four years. Sigh. -- BenRG (talk) 19:49, 3 November 2009 (UTC)
- It's only in a very limited way that quantum entanglement can violate locality. Regardless of quantum entanglement, information can't travel faster than the speed of light, which applies in the case of pushing a long rod. See no-communication theorem. Red Act (talk) 18:42, 3 November 2009 (UTC)
Billiard ball model becomes sort of invalid when you're talking about this timescale. Why are things rigid? Why do you not fall through the floor? Ultimately there is repulsion between the electron clouds of the floor and the electron clouds of your shoes/feet: an electromagnetic interaction. Indeed, chemical bonds are not rigid: they constantly oscillate (those oscillations get bigger with temperature). It takes time for this repulsion to occur and for it to be transmitted through a substance. (It's the fact that bonds are not rigid that allows for much of carbonyl chemistry and stabilised transition states.) John Riemann Soong (talk) 15:53, 8 November 2009 (UTC)
I think it is difficult to parse in the posit of my second lede but let suppose there is a theoretical ship of one light second long (ab) which can travel with its initial constant velocity say 10 km/sec. There are two persons A and B, a slight greater than a light second apart. A starts the theoretical ship at his end “a” towards B in order to wake him up. Since the distance between B and b is very very small as compared to ab or AB therefore would the end "b" of vehicle be reached B in less than second?
Two persons A_____________________B
Theoretical Ship a___________________b
68.147.38.24 (talk) 04:06, 9 November 2009 (UTC) khattak
hox and homeotic transformation
"I have a question/challenge on the homeotic gene page. Are HOX actually homeotic? They definitely are in their ability to regulate A/P development (with anteriorization if a paralogous cluster is knocked out), but there are not homeotic transformations of the limbs in knockouts (HOX 10 and HOX 11 Genes are Required to Globally Pattern the Mammalian Skeleton, Capecchi, et al., 2003, Science Vol 301, p363) From this article, while there are gross limb defects, there is not the reassignment of stylopod to zeugopod, or anything along those lines, making me think that these genes are not completely homeotic in nature. Thoughts? Thanks for maintaining this extremely important page." —Preceding unsigned comment added by Terragamo (talk • contribs) 05:06, 2 November 2009 (UTC)
- I believe the HOX genes are unusual in that respect due to the nature of their distinction in limb development. The differential expression of the HOX genes is established during the limb bud stage, when the future limb is at only a small fraction of its final length, barely extended from the imaginal disc. It'll be a day before I can get back to my old developmental textbook, though, so I should note that that's simply my idea. Someguy1221 (talk) 05:12, 2 November 2009 (UTC)
carbon monoxide
Plants metabolize carbon dioxide. What about carbon monoxide? 71.100.8.110 (talk) 06:20, 2 November 2009 (UTC)
- They're capable of metabolizing carbon monoxide, but the experiments that showed this exposed the plants to carbon dioxide free atmosphere, so I'm not sure how physiologically significant the effect is. One paper describing this: [2] Someguy1221 (talk) 06:26, 2 November 2009 (UTC)
What happens if a non-depressed person takes anti-depressants?
Not a request for medical advice as I'm just curious about what would happen. Assuming they took them regularly for some time. Would they feel happier, drunk, manic, lose their rational judgment, what? It seems like a scenario for a dystopian science-fiction novel. 78.146.167.26 (talk) 14:58, 2 November 2009 (UTC)
- The answer is going to depend on which specific anit-depressant drug is taken. Googlemeister (talk) 15:39, 2 November 2009 (UTC)
- And the dose. --Mr.98 (talk) 16:13, 2 November 2009 (UTC)
- Don't those drugs sometimes have nasty side-effects? RJFJR (talk) 15:47, 2 November 2009 (UTC)
- Drug abuse and antidepressant might be a good starting point... TastyCakes (talk) 16:25, 2 November 2009 (UTC)
- Terrible withdrawals as well if you forget a dose. Readro (talk) 16:56, 2 November 2009 (UTC)
- Not really. They take several days to begin to take effect. Withdrawal happens after a week or so.--Drknkn (talk) 17:30, 2 November 2009 (UTC)
- That really depends on the drug (and its half-life). Effexor can cause withdrawal with a single missed dose, the same day you missed it. -- Aeluwas (talk) 18:38, 2 November 2009 (UTC)
- I was speaking from personal experience, and you managed to guess my brand of pills in one! Congratulations :) Readro (talk) 20:53, 2 November 2009 (UTC)
- I didn't know about that drug. I've used Paxil, Prozac, and Zoloft. They work slowly.--Drknkn (talk) 21:15, 2 November 2009 (UTC)
- I was speaking from personal experience, and you managed to guess my brand of pills in one! Congratulations :) Readro (talk) 20:53, 2 November 2009 (UTC)
- That really depends on the drug (and its half-life). Effexor can cause withdrawal with a single missed dose, the same day you missed it. -- Aeluwas (talk) 18:38, 2 November 2009 (UTC)
- Not really. They take several days to begin to take effect. Withdrawal happens after a week or so.--Drknkn (talk) 17:30, 2 November 2009 (UTC)
- The vast majority of real-world side effects are mental. Lethargy (laziness), and apathy are common, especially with Zoloft. Anti-depressants do more to curb caring in general than depression. So, you don't feel over-joyed or anything. You'd need a stimulant (like, say, ritalin) for that. If you're already happy, and you take a stimulant, then you'd feel ecstatic. But if you're anxious and you take a stimulant, then the results could be catastrophic.--Drknkn (talk) 17:29, 2 November 2009 (UTC)
- [citation needed]. Comet Tuttle (talk) 18:04, 2 November 2009 (UTC)
- This article suggests that some non-depressed people have become habitual users of anti-depressants for "lifestyle" reasons, perhaps after first being prescribed them for reasons other than depression. The user cited in the article does indeed claim that his daily SSRI makes him feel happier, although it is made clear that the effects of using these drugs in this way are unknown and potentially dangerous. Karenjc 18:40, 2 November 2009 (UTC)
- I don't think it's that simple. Depressants like alcohol, for unknown reasons, make people feel good, while stimulants often dampen their enthusiasm. Imagine Reason (talk) 19:10, 2 November 2009 (UTC)
- Right. I guess it depends on your personality. I've used Paxil, Prozac, and Zoloft over the years. They all make me lethargic and apathetic. Alcohol makes me depressed, but other people get happy when they're drunk, so YMMV.--Drknkn (talk) 21:15, 2 November 2009 (UTC)
- [citation needed]. Comet Tuttle (talk) 18:04, 2 November 2009 (UTC)
- There are different types of anti-depressants. "Tri-cyclics", "MAOI", "SSRI", and then a bunch which are a bit like SSRIs but have different acronyms, such as NASAs, etc. Ann and Bob might have very different experiences even of the same dose of the same medication. There's something called "seretonin syndrome" which can affect people who overdose on soem types of anti-depressant meds. One way to answer the question would be to look at the side-effects of the medications, and the frequency of those side effects, to see what might happen. Don't forget the important Placebo Effect either. Remember Civility (talk) 19:33, 2 November 2009 (UTC)
- Some antidepressants are routinely prescribed for their side-effects, not their anti-depressant effect. See Amitryptiline. I've just come off this drug after 15 years on it for nerve pain: apparently it is the treatment of choice for such pain. I didn't notice a particular anti-depressant effect while I was on it. --TammyMoet (talk) 20:33, 2 November 2009 (UTC)
- The best conversations of this variety I've had focus on how little one might notice unless in a particular or exceptional situation, or unless they already knew what to look for. Regardless of type taken and mental state before starting, some things that are pretty generic side effects anyone could get could start to appear in just a few days if there's even a minor adjustment in what was already a good chemical balance. Things like... Misinterpretation of intoxication level , headache, light sleeping problems/problems falling asleep, loss of sex drive, difficulty concentrating, unusual fatigue/grogginess are universal. Those are all relatively minor and are pretty easily shrugged off by long-term patients but could feel quite amplified and frightening if you body doesn't quite know what to do with it. There aren't any specific "automatics" as theoretical benefits, and even if there were I can't see why someone would want to put up with the negatives. My biggest concern for someone in "normal" mental health using would be the risk of sudden, um... death? Without a doctor to look over your medical history and put you on a small trial dose of something to make sure you're not allergic in any way, odds are higher of running into some terribly bad side effects like an irregular heartbeat, confusion and blackouts (re: driving), general paranoia, insomnia. Even if none of that ever happened, you'd continuously be at the risk of day-to-day stresses or normal medications throwing things awry; Extreme caffeine consumption, certain cold or allergy medications, prescription painkillers or just extreme fatigue might trigger an undesirable reaction either 'up' or 'down'. This why there will never be any official studies-- it's just too damn unethical to ever have someone take medications like this "just to see". I'd thwap anyone who decided to so something this stupid. ♪ daTheisen(talk) 09:38, 4 November 2009 (UTC)
the form of energy as matter
Harmonic analysis/synthesis reveals that a combination of sinusoidal frequencies can generate an approximate square wave of any amplitude with a virtually infinite wavelength or zero frequency (assuming that in the case of the expression of energy that the reason absolute zero can not be obtained is the same reason absolute zero frequency can not be obtained). Is it possible that this state of combined frequencies is the state of energy we call energy or matter in the form of a particle? Biggerbannana (talk) 15:25, 2 November 2009 (UTC)
- I'm not really sure what you mean. What are you measuring the frequency of exactly?
- The wave function of a particle has a non-zero frequency. See matter wave. Rckrone (talk) 17:23, 2 November 2009 (UTC)
- The idea I am referring to is that of matter or energy being created in a star say by fusion when two atoms of hydrogen becomes one atom of helium. While hydrogen and helium are both matter the energy difference is not expressed in the form of matter but in the form of electromagnetic radiation. The amount of this electromagnetic radiation is known. However, the radiation contains both light and heat and x-rays and Gamma rays and more. However, if you only measure the visible light energy then you come up short and the same for all of the other wavelengths as well but not if you add them all together. When you do add them all together and plot a graph you should get approximately a square wave which itself may have a wavelength near one half the half life of Helium. What I am asking is has anyone else explored this idea and if so have they written a paper? Biggerbannana (talk) 20:52, 2 November 2009 (UTC)
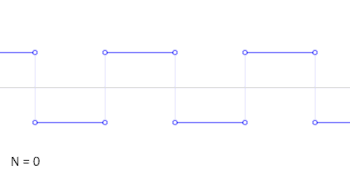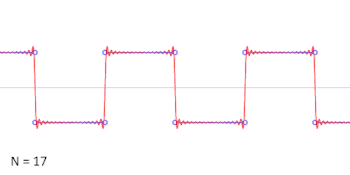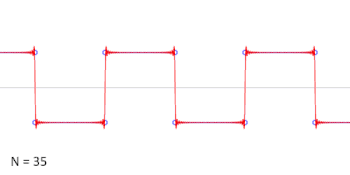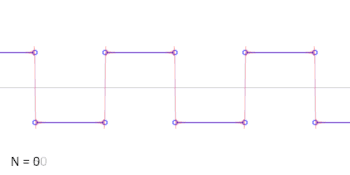
- A square wave is the sum of an infinite series of sinusoidal waves. Conversely a square wave can be generated by adding together the same series of sinusoidal waves. The frequency of the square wave is that of the lowest frequency sinusoidal wave, so this is not a way to generate a lower frequency wave than one already has. The amplitude of the square wave is less than the amplitude of the lowest frequency sinusoidal wave, shown by the animation. Cuddlyable3 (talk) 22:25, 2 November 2009 (UTC)
- What would say is the lowest electromagnetic frequency produced by the fusion reaction and its amplitude? For that matter how about the amplitude of each frequency produced? 71.100.8.110 (talk) 02:12, 3 November 2009 (UTC)
- A square wave is the sum of an infinite series of sinusoidal waves. Conversely a square wave can be generated by adding together the same series of sinusoidal waves. The frequency of the square wave is that of the lowest frequency sinusoidal wave, so this is not a way to generate a lower frequency wave than one already has. The amplitude of the square wave is less than the amplitude of the lowest frequency sinusoidal wave, shown by the animation. Cuddlyable3 (talk) 22:25, 2 November 2009 (UTC)
- A single fusion reaction doesn't create a wide spectrum of EM radiation. The energy released is in the form of just one or a few high energy photons and some kinetic energy. Rckrone (talk) 00:09, 3 November 2009 (UTC)
- Yes and the amplitude and frequency of each...? Have any been measured and added? Biggerbannana (talk) 02:27, 3 November 2009 (UTC)
- They certainly have been measured, but it depends on what reaction it is and on your reference frame. The point I was making is that the Fourier series of a square wave has countably infinite non-zero terms. You can't build it out of 1 or 3 or even 100 frequencies. Rckrone (talk) 06:09, 3 November 2009 (UTC)
- I disagree. Although not a perfect square wave only a few frequencies are necessary to produce a reasonable one with less that maximum amplitude but sufficient duration to trigger a programmable voltage sensor for instance. Biggerbannana (talk) 13:26, 3 November 2009 (UTC)
- But they don't make anything that looks like that. If you have a reaction that sends off one gamma ray, there's no way to argue that's something like a square wave, and the frequency is somewhere on the order of 1019 Hz, which is a bit off from the theorized half life of a proton which would be less than 10-41 Hz. Rckrone (talk) 17:51, 3 November 2009 (UTC)
- The fact that the sinusoidal components of a square wave have specific relative amplitudes and phases seems to be overlooked here.Cuddlyable3 (talk) 08:04, 4 November 2009 (UTC)
- But they don't make anything that looks like that. If you have a reaction that sends off one gamma ray, there's no way to argue that's something like a square wave, and the frequency is somewhere on the order of 1019 Hz, which is a bit off from the theorized half life of a proton which would be less than 10-41 Hz. Rckrone (talk) 17:51, 3 November 2009 (UTC)
- I disagree. Although not a perfect square wave only a few frequencies are necessary to produce a reasonable one with less that maximum amplitude but sufficient duration to trigger a programmable voltage sensor for instance. Biggerbannana (talk) 13:26, 3 November 2009 (UTC)
- Also not sure how you would measure the amplitudes of each of these components. The Fourier coefficients have to go to zero as the frequency go to infinity, but the energy of a photon grows. Rckrone (talk) 06:45, 3 November 2009 (UTC)
- They certainly have been measured, but it depends on what reaction it is and on your reference frame. The point I was making is that the Fourier series of a square wave has countably infinite non-zero terms. You can't build it out of 1 or 3 or even 100 frequencies. Rckrone (talk) 06:09, 3 November 2009 (UTC)
- Yes and the amplitude and frequency of each...? Have any been measured and added? Biggerbannana (talk) 02:27, 3 November 2009 (UTC)
- A single fusion reaction doesn't create a wide spectrum of EM radiation. The energy released is in the form of just one or a few high energy photons and some kinetic energy. Rckrone (talk) 00:09, 3 November 2009 (UTC)
BTW -thanks for whomever provided the graph... 71.100.8.110 (talk) 02:14, 3 November 2009 (UTC)
- YW. User Kief on Commons made the animation and I posted it. Cuddlyable3 (talk) 11:16, 3 November 2009 (UTC)
- Much thanks or hugs and kisses if you are female. ;) Biggerbannana (talk) 13:28, 3 November 2009 (UTC)
- That is the kind of male chauvinist sexist discrimination that belongs to another less enlightened age. Keep your banana to yourself.Cuddlyable3 (talk) 08:00, 4 November 2009 (UTC)
- Much thanks or hugs and kisses if you are female. ;) Biggerbannana (talk) 13:28, 3 November 2009 (UTC)
Definition of species
The most common definition of species that I've seen is that a species is a group of organisms that can breed with one another to produce fertile offspring. How are species defined for bacteria and other organisms that reproduce asexually? ----J4\/4 <talk> 18:15, 2 November 2009 (UTC)
- Species#Definitions of species discusses this issue. Basically, the answer is that there is no good answer. --Tango (talk) 18:30, 2 November 2009 (UTC)
- It's a controversial area. See "Species" and "Species problem". There are many species that have been renamed/reclassified: Helicobacter pylori (previously Campylobacter/campylobacter-like organism), Moraxella catarrhalis (previously Branhamella), Pneumocystis jiroveci (previously Pneumocystis carinii), etc.. Axl ¤ [Talk] 18:35, 2 November 2009 (UTC)
- Species in bacteria have been called 'fuzzy'.[3] Fences&Windows 03:17, 6 November 2009 (UTC)
- In reference to bacterial speciation, you may want to check out bacterial conjugation between 'species'. DRosenbach (Talk | Contribs) 18:00, 8 November 2009 (UTC)
Centrifugal force in Earth's orbit
Are there any observable effects of the centrifugal force experienced on the Earth as a result of its orbit around the sun? (Not the centrifugal force resulting from the spinning of the earth on its axis.) For instance, the weight of an object should be slightly greater when it is between the earth and the sun (i.e. daytime) versus when the earth is between it and the sun (i.e. nighttime). I assume that this weight discrepancy would be too small to measure, but is there any such effect that can be measured? Or perhaps I'm thinking wrong about the whole thing? Thanks- Staecker (talk) 19:56, 2 November 2009 (UTC)
- There is a tidal force on the Earth due to the sun that causes a noticeable effect. The tidal force on the Earth due to the moon is about twice as big, though. The tidal forces on the Earth from the moon and to a lesser extent the sun are what causes tides. Red Act (talk) 20:18, 2 November 2009 (UTC)
- Indeed. That tidal force is what the OP is describing - the centrifugal force is equal to the gravitational force (in an inertial frame of reference the gravitational force is a centripetal force and there is no centrifugal force, but if the Earth's frame there is). The difference between the gravitational force between two points on the Earth is called a tidal force. --Tango (talk) 20:27, 2 November 2009 (UTC)
- I'd just add that sometimes the Sun tide and Moon tide reinforce one another (the so-called spring tide), and sometimes partially cancel out (neap tide). This effect has presumably been observed for as long as Man has gone to sea, so yes, the effect is very observable, even if it took a while to figure out the reason. --Trovatore (talk) 20:29, 2 November 2009 (UTC)
- Tides have nothing to do with centrifugal force. Tides are due to gravity gradient, i.e. the fact that gravity diminishes with distance. Centrifugal force is due to being in a rotating frame of reference, i.e. in orbit. If the Earth was falling directly into the Sun instead of orbiting, there would by no centrifugal force due to its orbit, but there would still be a tide. Conversely, if the Earth was in a circular orbit but gravity was constant regardless of distance instead of diminishing as 1/r², there would be centrifugal force but no tides. --Anonymous, 06:50 UTC, November 3, 2009.
- No, that's not so. The centrifugal force is larger on the midnight side than on the noon side. That still gives you tides. --Trovatore (talk) 06:53, 3 November 2009 (UTC)
- Okay, that's a force gradient effect, but it's not a gravitational force gradient effect, therefore not a tide. --Anon, 22:49 UTC, November 3, 2009.
- Well, it'll make the oceans swell up, so whatever you wanna call it, I guess. From the point of view of Mach's principle it should be the same.
- A back-of-the-eyelids calculation last night while I was driving home suggests that the centrifugal force gradient should be responsible for one third of the observed tidal effect, not considering the Earth's rotation, which is an approximation I don't know whether you can make or not. Wouldn't stake my life on that being right, but it made sense while driving, anyway. --Trovatore (talk) 23:00, 3 November 2009 (UTC)
- It must be 100% of it, otherwise where does the rest come from in that rotating reference frame?
There is no gravitational force in that frame.Wait, that's not true. There is no resultant force in that frame. The centrifugal force must therefore equal the gravitational force, so presumably they each account for half the tides. --Tango (talk) 23:09, 3 November 2009 (UTC)
- It must be 100% of it, otherwise where does the rest come from in that rotating reference frame?
- Centrifugal force is the same as centripetal force, just in a different frame of reference (and in the opposite direction). With constant gravity you would have centrifugal force because you have centripetal force (the gravity). The centripetal force would be constant, therefore the centrifugal force would the constant, therefore there would be no force gradient effects from either force. You can only have tides due to varying centrifugal force if you have a varying centripetal force and it is distinguished from tides only by the frame of reference, so they are as equivalent as gravity and acceleration. You can consider a radial free-fall situation in a rotating reference frame and the centrifugal force will still be there, so if you really wanted to I expect you could reformulate the tides in that situation in terms of a varying centrifugal force (I haven't actually tried, though). --Tango (talk) 23:09, 3 November 2009 (UTC)
- No, there is no centrifugal force in this problem. I agree with Anonymous, tidal force has nothing to do with centrifugal force. A centrifugal force in this problem, if one existed, would tend to make the tides higher on the midnight side of the Earth than on the noon side of the Earth. But in reality, the tidal force raises the tides on the noon side of the Earth as much as on the midnight side of the Earth. Red Act (talk) 04:03, 4 November 2009 (UTC)
- To first order, the centrifugal-force gradient also raises the tide the same amount on both sides. The thing to keep in mind is that the two forces balance at the Earth's center. If the gravitational force were of constant magnitude, but centrifugal force behaved as it actually does, then that would still be true for a circular orbit (I'm not sure if the orbit would still be stable, but that's a separate question).
- So in this alternative reality with the constant gravity, we have that a piece of water in the noonday sun is subjected to the same gravitational force (from the Sun) as an equally massive piece singing Round Midnight. However the centripetal acceleration in daylight is smaller than that at the center of the Earth, which in turn is smaller by the same amount than that of the water gazing into the starry deep. --Trovatore (talk) 04:21, 4 November 2009 (UTC)
- (Oh, to clarify: This is still ignoring the Earth's rotation. Imagine for the moment that the Earth always keeps the same face to the Sun, just to simplify things.) --Trovatore (talk) 04:24, 4 November 2009 (UTC)
- Okay, that's a force gradient effect, but it's not a gravitational force gradient effect, therefore not a tide. --Anon, 22:49 UTC, November 3, 2009.
- No, that's not so. The centrifugal force is larger on the midnight side than on the noon side. That still gives you tides. --Trovatore (talk) 06:53, 3 November 2009 (UTC)
- The Effective potential is a way to rewrite the mechanical equations of motion to make it appear that earth's orbit is due to an "effective" force (centrifugal force) - but all that is really happening is that energy and momentum are being conserved. You can interpret the orbit as a "measurable effect" of centrifugal force in this treatment - but you should be aware of the assumptions made by such a formulation. Our effective potential article has a good overview of this topic. Nimur (talk) 20:33, 2 November 2009 (UTC)
- The centrifugal force you're thinking of doesn't exist here. If the Earth were being swung around on the end of a rope, you would feel yourself pressed toward the Earth if you were on the inner side and pushed away from it if you were on the outer side, and that difference would show up on a scale. But the Sun's gravity accelerates you along with the Earth, so there's no such effect. There is a much smaller effect due to the change of the gravitational field with distance (the tidal force already mentioned), but it's not the same thing. For one thing it's symmetrical—on the near side you're pulled away from the Earth because you're closer to the Sun, on the far side you're pulled away from the Earth because it's closer to the Sun. For another thing the tidal force drops off with distance from the Sun, while a centrifugal force would increase with distance. -- BenRG (talk) 20:46, 2 November 2009 (UTC)
Oh please, not this silly quibble again. http://xkcd.com/123 .Oops, sorry, you were making a different point; I hadn't read carefully enough. --Trovatore (talk) 20:48, 2 November 2009 (UTC)
- The weight will be greatest when the body is at right angles to the sun or moon, ie at dawn or dusk for the sun. Dmcq (talk) 20:49, 2 November 2009 (UTC)
- I think BenRG has the right response. I'm pretty sure that what I'm trying to talk about is different from the tidal force, which I know and understand perfectly well. My alleged force would have different properties than the tidal force (read my original description), but if I understand BenRG correctly this effect is cancelled by the sun's gravity. Staecker (talk) 20:54, 2 November 2009 (UTC)
- One way to think of it is that the Sun's gravity cancels the centrifugal force at the Earth's center. On the noon side of the Earth, the Sun's gravity is greater (because you're closer to the Sun) but the centrifugal force is smaller (because the angular velocity is the same, but the radius is smaller), so you're pulled towards the Sun. On the midnight side, the Sun's gravity is diminished, but the centrifugal force is larger, and pulls you out. --Trovatore (talk) 21:05, 2 November 2009 (UTC)
- ... of course this ignores the Earth's own rotation. The angular velocity on the noon side is not exactly the same as the angular velocity on the midnight side, because the Earth is spinning. I haven't worked out how much difference this makes. --Trovatore (talk) 21:44, 2 November 2009 (UTC)
- One way to think of it is that the Sun's gravity cancels the centrifugal force at the Earth's center. On the noon side of the Earth, the Sun's gravity is greater (because you're closer to the Sun) but the centrifugal force is smaller (because the angular velocity is the same, but the radius is smaller), so you're pulled towards the Sun. On the midnight side, the Sun's gravity is diminished, but the centrifugal force is larger, and pulls you out. --Trovatore (talk) 21:05, 2 November 2009 (UTC)
- I think BenRG has the right response. I'm pretty sure that what I'm trying to talk about is different from the tidal force, which I know and understand perfectly well. My alleged force would have different properties than the tidal force (read my original description), but if I understand BenRG correctly this effect is cancelled by the sun's gravity. Staecker (talk) 20:54, 2 November 2009 (UTC)
Mass spec
If I wanted to conduct an analysis of a suite of polycyclic aromatic hydrocarbons in a solvent extract of soil using mass spectrometry in order to obtain information on the molecular masses of the constituents what sort of ionisation and insertion techniques should be used? Thanks 188.221.55.165 (talk) 20:01, 2 November 2009 (UTC)
- I'm no analytic chemist, but it sounds like you're talking about GC-MS. Seems that's the way people are measuring soil contaminants these days (refs here: [4],[5],[6] will probably have more detail than WP:RD could provide). --- Medical geneticist (talk) 20:32, 2 November 2009 (UTC)
Banging your fists from a different perspective
The act of banging your fists on a table, a relatively common gesture, is usually displayed when someone wants to give their speech a firm and absolute meaning, or when that person is angry. What purpose does this gesture serve from an evolutionary perspective, that is, it obviously serves a purpose nowadays to humans, but is this gesture seen in other primates, and how does this gesture in particular serve us for a certain purpose, instead of using a different gesture. Any thoughts? —Preceding unsigned comment added by 201.21.180.57 (talk) 20:43, 2 November 2009 (UTC)
- As a layman, I'd suggest that banging one's fist on the table is no more "innate" than rolling one's eyes; our article Gesture is woefully short but has some interesting-looking references. Comet Tuttle (talk) 20:47, 2 November 2009 (UTC)
- Why make the assumption that this gesture must have an evolutionary "purpose" different than any other gesture? Fist thumping is a useful, easily interpreted gesture. Various primates (including humans) pound their fists on their chests... probably something having to do with trying to establish dominance or display aggression, etc. --- Medical geneticist (talk) 21:53, 2 November 2009 (UTC)
- I'm not sure how biologically relevant it is (I can't find a WP article), but stereotypically male gorillas are thought to pound their chests with their fists in a gesture of physical dominance. Pounding the table could be a related gesture used to connote power and authority. -- 128.104.112.149 (talk) 22:06, 2 November 2009 (UTC)
- Pounding with clenched fist an object such as a table is expressing an urge to Violence that is in conflict with one's unwillingness to hurt a person. Cuddlyable3 (talk) 22:10, 2 November 2009 (UTC)
- See the article Frustration noting the examples. Cuddlyable3 (talk) 07:55, 4 November 2009 (UTC)
- Not a good reference, as that paragraph not only doesn't support your claim here, but it has no references and is a mass of OR. Comet Tuttle (talk) 18:37, 6 November 2009 (UTC)
Can the Yarkovsky effect be used for space propulsion?
Say you build a probe that heats only part of its exterior using an internal radioactive source. Would you be able to get a net thrust out of this system because of the Yarkovsky effect? I looked around the net for things about such a mechanism but didn't really find anything. 189.15.218.71 (talk) 23:29, 2 November 2009 (UTC)
- Heating only part of the probe would be hard. You would be better off heating all of it and painting different sides different colours so they emit differently (white on one side so it doesn't emit much, black on the other so it does). That kind of thing has been proposed for giving asteroids a slight nudge over a long period of time so they don't crash into the Earth. I've never seen it proposed for spacecraft propulsion. I guess it would work, but it would be really slow - there are better ways. --Tango (talk) 23:42, 2 November 2009 (UTC)
- The problem is that radiation pressure at any reasonable temperature (i.e., a temperature low enough to not melt the propulsion system) is very small, generally measured in micropascals. So plenty of energy may be available from the radioactive source, but you're limited as to how fast you can use that energy, i.e., how much power the propulsion system can provide. That's why nuclear electric rocket designs instead use some kind of electric propulsion system such as an ion thruster, that uses some kind of reaction mass. Red Act (talk) 00:48, 3 November 2009 (UTC)
November 3
Mil vs Micron
Hi guys, Mil and Microns are measurements used to represent the thickness of plastic. Does any body knows how many microns are equal to 0.55 Mil? —Preceding unsigned comment added by Ferchyn (talk • contribs) 01:14, 3 November 2009 (UTC)
I would guess "Mil" is short for "millimetre" (one thousandth of a metre). A micron is an alternative name for a micrometre (one millionth of a metre). So there are 1000 microns in a mill. That means 0.55 Mil is 550 microns.And I would guess wrong - it's short for milli-inch. So the actual answer is 0.55 Mil is 13.97 microns. --Tango (talk) 01:25, 3 November 2009 (UTC)- (edit conflict) I was going to correct Tango, but now I don't need to. Our article is at Thou (length). Deor (talk) 01:33, 3 November 2009 (UTC)
- Hmm, in my experience mil is much more common. Probably the article should be moved. --Trovatore (talk) 03:07, 3 November 2009 (UTC)
- I agree, I've never even heard of thou. I'm sure mil is much more common. Red Act (talk) 03:13, 3 November 2009 (UTC)
- Thou is more common in my experience. Perhaps it is a British vs American English thing (me being British)? --Tango (talk) 03:19, 3 November 2009 (UTC)
- I agree, I've never even heard of thou. I'm sure mil is much more common. Red Act (talk) 03:13, 3 November 2009 (UTC)
- Hmm, in my experience mil is much more common. Probably the article should be moved. --Trovatore (talk) 03:07, 3 November 2009 (UTC)
- e/c Try this Mil to Micron conversion calculator. hydnjo (talk) 01:32, 3 November 2009 (UTC)
- (edit conflict) I was going to correct Tango, but now I don't need to. Our article is at Thou (length). Deor (talk) 01:33, 3 November 2009 (UTC)
- Worth noting is that Google supports unit conversions in their search field: Check it out. TenOfAllTrades(talk) 03:03, 3 November 2009 (UTC)
- If thou is a British thing, then why don't we have the article at Mil (length), since the British use the metric system? —Akrabbimtalk 04:28, 3 November 2009 (UTC)
- Should be, "if thou art a British thing". HTH. --Trovatore (talk) 04:30, 3 November 2009 (UTC)
- If thou is a British thing, then why don't we have the article at Mil (length), since the British use the metric system? —Akrabbimtalk 04:28, 3 November 2009 (UTC)
- Brits use a mixture of metric and imperial. If I need to gap a spark plug I know it's 25 thou, and I always used to gap distributor points to 15 thou. I wouldn't know (although could calculate or look up) the metric equivalent. We mostly inflate tyres to PSI and we always drive miles at MPH. Not very metric, really. --Phil Holmes (talk) 10:47, 3 November 2009 (UTC)
- Oh, I didn't know that. Here in America, all the sciencey people that like metric spin the US as the last barbaric nation to still hold on to feet, pounds, and gallons. —Akrabbimtalk 12:10, 3 November 2009 (UTC)
- No, there are 2 barbaric nations left ;-) Fribbler (talk) 13:19, 3 November 2009 (UTC)
- Quite recently I was remarking to my wife how I have to mix units to understand the sizes of things without lots of consciuos thought. I only really know my weight in stones and pounds (which, I know, won't help in the US). I run a weather site and follow rain in mm and temperature in Celsius. I'm equally at home with metres or feet or yards. I can only think of fuel consumption in miles per gallon. I used to work on Integrated circuits and couldn't conceive of specifying them in anything other that microns - oh - except their diameter, which is inches. Such is life :-) --Phil Holmes (talk) 17:21, 3 November 2009 (UTC)
- Hmm, you did do your silicon work a while ago, eh? The micron is still used (in the sense that no one says micrometer) but it's kind of too big to be a very useful unit. Sometimes comes up when talking about regions of a chip and stuff like that. --Trovatore (talk) 19:11, 3 November 2009 (UTC)
- Quite recently I was remarking to my wife how I have to mix units to understand the sizes of things without lots of consciuos thought. I only really know my weight in stones and pounds (which, I know, won't help in the US). I run a weather site and follow rain in mm and temperature in Celsius. I'm equally at home with metres or feet or yards. I can only think of fuel consumption in miles per gallon. I used to work on Integrated circuits and couldn't conceive of specifying them in anything other that microns - oh - except their diameter, which is inches. Such is life :-) --Phil Holmes (talk) 17:21, 3 November 2009 (UTC)
- No, there are 2 barbaric nations left ;-) Fribbler (talk) 13:19, 3 November 2009 (UTC)
- Oh, I didn't know that. Here in America, all the sciencey people that like metric spin the US as the last barbaric nation to still hold on to feet, pounds, and gallons. —Akrabbimtalk 12:10, 3 November 2009 (UTC)
- Brits use a mixture of metric and imperial. If I need to gap a spark plug I know it's 25 thou, and I always used to gap distributor points to 15 thou. I wouldn't know (although could calculate or look up) the metric equivalent. We mostly inflate tyres to PSI and we always drive miles at MPH. Not very metric, really. --Phil Holmes (talk) 10:47, 3 November 2009 (UTC)
Best time to buy new houseplants?
I'm a beginning apartment gardener who's ready to move beyond philodendron and spiderplants. I've purchased and read a number of guides and feel ready to buy some new species specimens. I'd like to know if there are guidelines as to the best time to purchase new plants? None of my books have mentioned this, other than notes about protecting plants from cold/heat damage during transit from your nursery to your home. For example, I was wondering if buying plants in spring, when they're waking up and preparing for new growth, makes them more able to adapt to the climate difference in their new location? I'd rather not buy a nice selection of new plants now only to find that they've been too weak to successfully acclimate to their new home... I have an underfloor heated apartment in a climate roughly equivalent to Ohio. Thank you! 218.25.32.210 (talk) 01:29, 3 November 2009 (UTC)
- Your indoor plants are going to have survive dry air conditions. It probably does not matter much when you buy them, but you may get a better price in the autumn when nurseries try to get rid of plants before frost kills them. Graeme Bartlett (talk) 08:29, 3 November 2009 (UTC)
- You'll get a better answer if you just tell us the species you want to grow indoors. One thing you might consider is setting up a heated propagation area (using a heat mat, plastic hoods or vivarium-like enclosures) and instead of buying plants, just grow some from seed, choose the best one as a mother plant, and clone the rest with cuttings. You can do this with several different species, and this will allow you to have some redundancy; If one plant dies, you'll still have the others to work with. Viriditas (talk) 11:05, 3 November 2009 (UTC)
- I actually find it better to buy the plants that are in flower when they are in flower, so I can see whether I like them or not! In the UK, houseplants in garden centres are generally on sale in heated greenhouses or similar environments, so they shouldn't need to acclimatise. --TammyMoet (talk) 11:30, 3 November 2009 (UTC)
Elements formed by a hydrogen bomb detonation
I've looked on many articles relating to nuclear processes but I haven't found anything particularly straight-forward on the elements created by an atomic explosion, especially hydrogen bombs, which produce high atomic mass elements. Could someone help find/add these elements and add them to an existing article? Much appreciated! Letter 7 it's the best letter :) 01:55, 3 November 2009 (UTC)
- I think the high atomic mass elements will be from the fission part of the bomb (ie. the bit that doesn't involve hydrogen). Nuclear fission product should contain the information you want, but note there aren't specific elements produced - there will be a mixture. --Tango (talk) 02:01, 3 November 2009 (UTC)
- Much obliged! In my rush I didn't see that page, thanks again! Letter 7 it's the best letter :) 02:09, 3 November 2009 (UTC)
- Note that there are some elements that are formed by nuclear reactions other than fission in a bomb. See, e.g., Einsteinium, which is formed by the capture of 15 neutrons by U-238 (which is the sort of thing that you'll only find in a very high neutron economy, of course—like the inside of a bomb, or a particle accelerator). --Mr.98 (talk) 04:41, 3 November 2009 (UTC)
composite spectral waveform of each element
Have the composite waveforms (harmonic synthesis) of the frequencies and amplitude of the spectral lines for each element been published? Biggerbannana (talk) 05:41, 3 November 2009 (UTC)
- What spectral line are you referring to ? If you mean the emission spectrum, you can find such data at (say) this NIST website. Abecedare (talk) 05:59, 3 November 2009 (UTC)
- Yes, this page seems to point to the spectral data but without graphs and in particular the waveform of the composite emission spectrum for each element. Biggerbannana (talk) 06:18, 3 November 2009 (UTC)
- The wavelength of the composite waveform is the Least common multiple of its component wavelengths and likely to be extremely long i.e. of low frequency. Cuddlyable3 (talk) 11:05, 3 November 2009 (UTC)
- Yes, this page seems to point to the spectral data but without graphs and in particular the waveform of the composite emission spectrum for each element. Biggerbannana (talk) 06:18, 3 November 2009 (UTC)
- The composite waveform does not really exist. One atom will emit one photon of a particular colour, and the waveform will depend more on how you measure it. Different colours from an aggregate of atoms will be emitted incoherently and will not be in phase with each other. This will mean there is not a particular wave shape. The overall spectrum will vary with temperature, pressure, magnetic field and turbulence, and even electric field. Graeme Bartlett (talk) 10:36, 4 November 2009 (UTC)
DIY amatuer water quality testing for real nasties like mercury, et al ?
Is it possible for someone without a mass spectrometer // well-equipped laboratory to test water quality for things beyond pH and dissolved oxygen levels? Are there kits one can buy that would reliably identify the presence of heavy metals and such? Or is the only recourse to send a sample to a lab? 218.25.32.210 (talk) 05:48, 3 November 2009 (UTC)
- I've seen at home test kits for mercury, lead, and nitrates. Also things like water hardness and sodium content, but those have less to do with whether the water is potentially harmful. Dragons flight (talk) 06:02, 3 November 2009 (UTC)
- Yes. Aquarium hobbyists measure a range of variables in the aquarium water. These are either test-strips or fluids you mix with water samples and they change colour depending on the concentration of various chemicals. These are available from websites and pet stores. Checking the website of JBL I find test sets for: Iron, copper, ammonium, phosphate, Nitrite, Nitrate, silicic acid, CO2 concentration and Gh and Kh measures of hardness, plus separate calcium measures and a magnesium + calcium indicator.
- I also know that in parts of india, the groundwater is tested for arsenic with similar and supposedly inexpensive equipment. EverGreg (talk) 09:49, 3 November 2009 (UTC)
- Mass spec isn't usually used for testing heavy metals in water. Inductively coupled plasma spectroscopy is more common. Rmhermen (talk) 15:26, 3 November 2009 (UTC)
aluminum-zinc alloys
Help! I'm trying to find a phase diagram for this system and am getting confused. In the first google images hit I find, I get alpha and alpha prime being in the same phase region?! Help?! John Riemann Soong (talk) 06:16, 3 November 2009 (UTC)
Information on shoulder/chest area anatomy (Medical Science)
Hi, I am writing a novel and I'm trying to make it as realistic as possible (sometimes to a brutal extent). I've come to a point where I need in depth medical information and my local doctor is indisposed. It's also tough to gleam the information I need from several (incomplete) diagrams. So here we are.
Be forewarned, the information I need is for a particularly descriptive(/brutal/violent) fighting scene.
I need to know if there is a name for the area between the shoulder and neck, that is to say, between the Clavicle and Scapula, as this is the point of entry of the character in question's sword. That would be the first part of my question. The second being: I also need a listing of tendons, muscles and organs that a 50cm sword would puncture/cut, if such an action was possible (Not obstructed by bones, etc). Or a source where I can get this. It may carry more relevance than simply determining whether the heart will be among this list, so I will include that it is on the right side of the individual. If the lungs are among this list (Which I believe it will be), am I correct in assuming the individual will cough or gurgle blood in his final moments?
Thank you in advance (In case this question isn't appropriate on this format, I do apologize. I must admit it was rather unclear to me at the time of this posting.) —Preceding unsigned comment added by RyuGenkai (talk • contribs) 09:52, 3 November 2009 (UTC)
Well, that's a nearly 20 inch sword. You can cut anything within 20 inches of where you stab, by aiming at it. Straight down, on the right, you'd hit the apex of the lung, the lung, the diaphragm and probably reach also have the liver en brochette. On the left, the apex of the lung, the pericardium, the heart, the diaphragm and the stomach. Should you decide to go sideways, you could probably get a lung-heart-lung shishkabob. You could cause bilateral pneumothorax and cause death by suffocation without any coughing or gurgling of blood, or if it make for a more dramatic scene you could have blood coughed everywhere. You could transect the carotid and have blood spurting out of the neck, or for a more subdued and dignified death, transect the aorta within the chest cavity and have the victim bleed to death internally with no mess on the carpet. Which is to say: big murder weapon can cause about anything entering about anywhere. Anyway, you should have a look at apex of the lung, which is the area you're entering, and you may get some idea of the anatomy of the area by looking at sternocleidomastoid muscle, scalene muscles, and File:Musculi_coli_base.svg. - Nunh-huh 10:15, 3 November 2009 (UTC)
- If I recall correctly, in Rome (TV series) Marcus Tullius Cicero was assassinated "execution style" by veteran soldier Titus Pullo (Rome character), who killed him with a downward sword thrust similar to the one you describe, as if it were a standard way of killing. In actual history, this is not documented. Edison (talk) 14:32, 3 November 2009 (UTC)
Thanks folks. Yes, I did mean straight down, don't know why I omitted that. I did say 50cm, but I'm assuming even with the momentum the character has (He's jumping down from above his victim) he won't bury it up to the hilt. At least not with the (limited) knowledge I have of Physiology. I've always had the opinion that humans are a lot more resilient than books and movies make them out to be. I am liking the pneumothorax idea - it opens up new options. I must admit I also have never heard of a historical case of killing in this way, but then again, my character isn't much for history. Nor is he very experienced in the killing business, shall we say. This is the best method he could come up with when presented with a drop from elevation onto the target. RyuGenkai (talk) 16:14, 3 November 2009 (UTC)
- What sort of author capitalizes common nouns like 'physiology,' 'clavicle' and 'scapula.' DRosenbach (Talk | Contribs) 20:43, 3 November 2009 (UTC)
- One whose first language is German? Googlemeister (talk) 15:38, 4 November 2009 (UTC)
- The film Torn Curtain is notable for a murder scene "that Hitchcock made specifically to show the audience how difficult it is to kill a man". 81.131.65.113 (talk) 21:38, 3 November 2009 (UTC)
melanin benefit vs cancer risk
Melanin seems to prevent damage to DNA by keeping free radical generation at a minimum. Its deficiency appears to be associated with genetic abnormalities. Is there a "Goldy Locks" level (not too hot and not too cold) of melanocytes to maximize benefit and minimize risk tht can be achieved through selective breeding? Biggerbannana (talk) 13:18, 3 November 2009 (UTC)
- I assume that would vary by the exposure in question, right? That's why melanin content varies in human populations by latitude. The averages of human skin pigments of historic populations at given latitudes is probably close to an ideal "level" for that given latitude, with evolution having found the sweet spot for that level of exposure. --Mr.98 (talk) 13:41, 3 November 2009 (UTC)
- Actually I mean amount of melanocyte cells rather than melanin assuming more cells produce more melanin in total. Biggerbannana (talk) 13:49, 3 November 2009 (UTC)
- Our articles on melanin and melanocyte don't appear to mention any association with genetic abnormalities. Do you have some source for that could be used to improve the articles? 75.41.110.200 (talk) 15:16, 3 November 2009 (UTC)
- The very article you linked to says "The difference in skin color between fair people and dark people is due not to the number (quantity) of melanocytes in their skin, but to the melanocytes' level of activity (quantity and relative amounts of eumelanin and pheomelanin). This process is under hormonal control, including the MSH and ACTH peptides that are produced from the precursor proopiomelanocortin." I didn't know this for sure before I read the article but expected it would be very likely for there to be a big difference in the regulation outside the number of cells as many/most? human systems have rather complex regulation. Nil Einne (talk) 16:20, 3 November 2009 (UTC)
- Has now been by blocked User:TenOfAllTrades, check WT:RD for more. Nil Einne (talk) 16:26, 3 November 2009 (UTC)
- Actually I mean amount of melanocyte cells rather than melanin assuming more cells produce more melanin in total. Biggerbannana (talk) 13:49, 3 November 2009 (UTC)
How is heat transfered from one body to another?
and no not a homework question, I assumed it was by the vibrating or moving particles in the hotter substance banging off the particles in the cooler substance/the air in between and causing them to vibrate or move as well. Is that true? —Preceding unsigned comment added by 92.251.255.16 (talk) 17:04, 3 November 2009 (UTC)
- Start with our heat transfer article. DMacks (talk) 17:07, 3 November 2009 (UTC)
- The OP describes heat conduction. The other two ways of heat transfer are radiation and convection. Cuddlyable3 (talk) 20:05, 3 November 2009 (UTC)
- Sometimes - and to some degree, yes - it's true - but it's not the only way.
- The molecules in the hot object are jiggling around - if they bash into the molecules of some cooler object (which aren't jiggling around as much) then some of the motion gets transferred so the hot object jiggles less than it was before and the cool object jiggles more than it did before - in other words, heat flowed from the hot object to the cooler one. That's "conduction".
- However, there is a second mechanism - hot objects emit infra-red light (or visible light if they are VERY hot..."glowing red hot" as we would say). That energy is lost from the hot object - and when that infra-red light hits something else, that object gains energy and heats up. That's how you can feel the heat from a fire - even though the air between you and the fire isn't all that hot. This is "radiant heat transfer".
- The other mechanism that people talk about is "convection" - which really only happens in fluids (gasses and liquids essentially) that are in contact with some hotter or cooler material. A pocket of fluid that's in contact with some hot object will get warm (by either conduction or radiation) - and that hotter region of the fluid expands and becomes less dense - which causes it to rise up and be replaced by cooler fluid that circulates in to replace it. As that hot pocket of fluid moves upwards, some cooler fluid flows in and is now in contact with the hot object and can remove more heat as it too expands, moves upwards and is replaced. The moving fluid (be it gas or liquid) carries the heat away - and can transport it faster than mere conduction through the fluid would be able to.
- If conduction were the only mechanism, then heat from the sun would be unable to reach us because of the 93 million miles of vacuum between there and here!
- But these are only partial descriptions. The hot flame from a coal fire can boil water, the steam can turn a generator, electricity can carry the energy away - be stored in a battery for six months - then used to spin a motor - whose friction produces heat. In a sense, this is yet another heat transfer mechanism. However, classically we talk about conduction, radiation and convection as being the only three ways it happens. Personally, I don't think convection counts - it's just the consequence of either conduction or radiation (and probably both)! However, if this actually IS homework - you'd better mention convection and not boiling-water/steam/generator/electricity/battery/motor/friction or any other of the bazillion ways heat is "transferred" - because that's just how homework is.
- SteveBaker (talk) 04:10, 4 November 2009 (UTC)
- Furthermore, convection is complicated in that it actually involves 2 processes; the transfer of energy from molecule-to-molecule via collisions, AND mixing of molecules of different energies. Since temperature is a bulk property of matter, I can have two theoretical systems:
- The first system has 50% of its molecules at energy = A; and 50% and energy = B
- The second system has 100% of its molecules at energy = C; such that C is the average of A and B
- Both of these systems are at the same temperature, however both are very different thermodynamically; the first system is at a higher entropy for example. How each of these systems react when brought into contact with a third system is very different; and yet since temperature is the ONLY practical means we can measure thermal energy, it makes these issues in detail much more complex than they seem in general. --Jayron32 21:27, 4 November 2009 (UTC)
- Furthermore, convection is complicated in that it actually involves 2 processes; the transfer of energy from molecule-to-molecule via collisions, AND mixing of molecules of different energies. Since temperature is a bulk property of matter, I can have two theoretical systems:
What species is the mushroom in this photo?
I have taken a set of five photos of some mushroom I found in the coastal forest of Poland in October 2009. I think the images have high value but I do not know the type of mushroom in the photos. I have five images in the total set. Here is one of them File:Unidentified_red_mushroom_in_Poland_in_October_2009.jpg and I can post more if need to help identify it. Jason Quinn (talk) 18:00, 3 November 2009 (UTC)
- Amanita muscaria or fly agaric --Tagishsimon (talk) 18:07, 3 November 2009 (UTC)
- That was the first species I found but I don't think it is correct. The mushrooms in my photos (there are the two you see in this photo and a third one in pictures I haven't posted yet) are flatter and less bell-shaped. Jason Quinn (talk) 18:10, 3 November 2009 (UTC)
- Actually, maybe you are correct. Some of the other pictures of them show them to flatter sometimes. I'll wait for more opinions. Thank you. Jason Quinn (talk) 18:12, 3 November 2009 (UTC)
- That was the first species I found but I don't think it is correct. The mushrooms in my photos (there are the two you see in this photo and a third one in pictures I haven't posted yet) are flatter and less bell-shaped. Jason Quinn (talk) 18:10, 3 November 2009 (UTC)
- If you Google image amanita muscaria there is a surprising number of forms displayed. I can't believe that the labelling is so bad. The other point to note is that the cap changes shape as it ages.It opens from an egg shape and will flatten as it matures, some even curling up at the edge, in the final stages before they decompose. Richard Avery (talk) 18:22, 3 November 2009 (UTC)
- Coming here from WikiProject Fungi, that's almost certainly a fly agaric. Our article is already very well illustrated, but a few more in the Commons gallery couldn't hurt. J Milburn (talk) 10:20, 4 November 2009 (UTC)
- If you Google image amanita muscaria there is a surprising number of forms displayed. I can't believe that the labelling is so bad. The other point to note is that the cap changes shape as it ages.It opens from an egg shape and will flatten as it matures, some even curling up at the edge, in the final stages before they decompose. Richard Avery (talk) 18:22, 3 November 2009 (UTC)
-
Photo 1 of 5
-
Photo 2 of 5
-
Photo 3 of 5
-
Photo 4 of 5
-
Photo 5 of 5
- Based on the feedback I received. I have uploaded my set of five photos to the Wikipedia Commons with the mushroom identified as amanita muscaria. The gallery above shows the images. Note that in image 4 you can clearly see the gills under the cap on the mushroom on the right, which may help you experts with the classification even more. Strangely, 4 of the 5 pictures show flies standing on the mushroom but the Wikipedia page says the mushroom is poisonous to flies. Either these flies have a death wish or something else is going on. Jason Quinn (talk) 14:28, 4 November 2009 (UTC)
Noon in the tropics
Would the variation of the position of the sun, at noon, in the tropics be noticeable. Presumably at some times of the year it is due north, and at others due south. Would it be noticeable? Stanstaple (talk) 18:26, 3 November 2009 (UTC)
- Yes. My Dad used to live in Kuala Lumpur (latitude 3 degrees north) and his condominium had a swimming pool. The temperature of that pool varied widely at different times of the year because the sun would go one side of the building at one time of year and the other side 6 months later. That means that during dry season the swimming pool spent quite a long time in the shadow of the building and during wet season it was in the sun almost the whole time, so was much warmer during the wet season. --Tango (talk) 18:51, 3 November 2009 (UTC)
- Just as in other places, the position of the sun in the sky at the same time of day has range of about 47° (twice the tilt of the Earth's axis) depending on the time of year. Rckrone (talk) 20:14, 3 November 2009 (UTC)
- 47 degrees doesn't really mean anything to me viscerally- i very much notice that the day is shortening dramatically at my latitude- the day goes from bright from four till eleven to eight till four- i just wanted to compare Stanstaple (talk) 21:58, 3 November 2009 (UTC)
- The length of the day doesn't change considerably in the tropics because the Sun goes from 23.5 degrees one side of directly overhead to 23.5 degrees the other side. --Tango (talk) 22:45, 3 November 2009 (UTC)
- 47 degrees doesn't really mean anything to me viscerally- i very much notice that the day is shortening dramatically at my latitude- the day goes from bright from four till eleven to eight till four- i just wanted to compare Stanstaple (talk) 21:58, 3 November 2009 (UTC)
ptsd
if you had ptsd how long does it last —Preceding unsigned comment added by 24.98.148.83 (talk) 19:43, 3 November 2009 (UTC)
- You mean Posttraumatic stress disorder? According to Posttraumatic stress disorder#Diagnosis, it has to last more than a month to count as PTSD. I think it can last the rest of someone's life in some cases. --Tango (talk) 19:49, 3 November 2009 (UTC)
- OR my father had PTSD arising from his treatment in a Japanese POW camp during WW2. It lasted until the day before he died 60 years later. Of course it was never treated, as PTSD was only recognised as a treatable condition comparatively recently. --TammyMoet (talk) 10:44, 4 November 2009 (UTC)
Glitch in thermochemistry calculation
Okay, so I have this little problem:
- A 100-gram rod of copper (specific heat 0.385 J/g) at 100ºC is immersed in 50 grams of water (specific heat 4.18 J/g) at 26.5ºC. What will the temperature be when both components achieve thermal equilibrium?
And I have tried to solve it as follows:
- From the information given, we can calculate that when copper cools by 1ºC, it releases 38.5 joules. On the other side, water needs 209 joules to gain 1ºC.
- Therefore, we can produce the following equations:
- and
- where x represents the amount of joules gained/lost by the component, and y represents the temperature of the component.
- To know where the equations intersect, we make them equivalent:
- We simplify:
- Now that we know that each component has gained/lost 2,371 joules, we can use that in the original equation to find out the gain/loss of temperature. Let's do it for copper:
- Therefore, that means that copper has lost 61.59ºC, i.e. its temperature at equilibrium is 38.41ºC. Nevertheless, my textbook gives a value of 37.9ºC. Did I make a mistake, or is this small deviation due to differences in rounding up/down numbers? Thank you. Leptictidium (mt) 19:52, 3 November 2009 (UTC)
- I think you made an error simplifying - I get x=2389.566667, albeit on the back of an envelope and as yet unchecked.
--Tagishsimon (talk) 20:17, 3 November 2009 (UTC)
- I'm sorry for asking what is probably a very stupid question, but where does the 8046.5 come from? Leptictidium (mt) 20:48, 3 November 2009 (UTC)
- It's 38.5*209 ... just some convenient figure allowing me to get rid of both fractions. --Tagishsimon (talk) 20:51, 3 November 2009 (UTC)
- I'm sorry for asking what is probably a very stupid question, but where does the 8046.5 come from? Leptictidium (mt) 20:48, 3 November 2009 (UTC)
Technically specific heat is temperature-dependent -- heat capacity falls as temperature decreases (it is zero at 0K). If you have 1 mol copper at 0C and 1 mol copper at 100C, their equilibrium temperature will actually be higher than 50C, because for each joule of heat transferred, the colder object increases in temperature faster than the hotter object falls in temperature per joule of heat. (This is what makes heat transfer from hot to cold thermodynamically irreversible.) John Riemann Soong (talk) 16:43, 4 November 2009 (UTC)
- Yes, but that level of accuracy isn't usually taught to first-year chemistry students, with good reason. First, one must understand the concept of specific heat before one can deal with the issue of temperature dependence of specific heat. The OP does not indicate what chemistry class he is taking, but it is likely this is simply general chemistry. --Jayron32 21:19, 4 November 2009 (UTC)
- Note: There is also another way of working it out
- 1. 100g copper going from 100°C to 26.5°C would release 100*0.385*(100-26.5) = 2829.75 J
- 2. Sum the copper and Water heat demands (also known as a "Water Equivalent") 50*4.18 + 100*0.385 = 247.5 J/K (wt * Cp = g * J/g/K = J/K) (Cp units are J/g/K not J/g as you shown above)
- 3. Divide (1) by (2) for the temp rise for the combined copper/water system 2829.75/247.5 = 11.4333K
- 4. Add (3) to the start temp = 26.5 + 11.4333 = 37.9333°C
- Note: There is also another way of working it out
looking at yellow or bright purple stuff for a long time
From my personal observence, okay I wear yellow goggles at night when I sleep. When I wake up when I take off the yellow goggle I still see white stuff for white. If humans look at yellow or purple stuff for a long time I thought white stuff will stay white, and it will not look blue or lime green. Since when I took off the yellow goggle I didn't see white as blue. Will yellow and purple just look less vivid? --209.129.85.4 (talk) 20:22, 3 November 2009 (UTC)
- The human brain compensates for ambient light (wearing yellow goggles would be the same as having the room illuminated by a yellow light). You know what things ought to be white and your brain works out what the ambient light must be and then compensates for it so you see things the colours they would be under white light. If you were in unfamiliar surroundings and didn't know what colours things should be, then your brain might get it wrong or it may take some time to work it out. --Tango (talk) 20:34, 3 November 2009 (UTC)
- Why do you wear yellow goggles when you sleep? Looie496 (talk) 20:39, 3 November 2009 (UTC)
- I do not think that the brain will know that you are wearing yellow goggles when you sleep because your eyes are going to be closed and it will presumably be dark. What colors do you think there are that need compensation under those conditions? Googlemeister (talk) 15:34, 4 November 2009 (UTC)
UFO identification
|
This question inspired an article to be created or enhanced: |
I snapped these beautiful critters on Sunday at the Kirstenbosch National Botanical Garden. Can anyone please help identify them? Also, please advise if these pics are the best of their type and pick the best ones between the duplicates. I'm loathe to upload the full res versions if we already have better pictures in our articles. Also, anyone with a good eye for composition feel free to crop these photos (or even just draw borders on the existing) as you see fit. The full res pics are 8MP so I should be able to throw away plenty of pixels and still have something usable.
Photographic critique would be greatly appreciated. Also what's the best way to go about editing these? They are taken with a Canon EOS 350D in Adobe RGB color space, will The GIMP handle it properly? (The low-res versions have been created with Windows Image Resizer PowerToy, causing some colour "bleeding" which I put down to it not playing nice with Adobe RGB.) Or should I just upload the originals and request our WP:Graphic Lab to do the touching up? Regards. Zunaid 20:37, 3 November 2009 (UTC)
- Dropped by further to message on the WP:photo page. Don't have enough time to upload crops, but here's my 2¢... calling them #1 to #7, clockwise from top left: 1 is a good in-flight shot and more worthy of gallery space than others there, crop slightly (15%) from all sides except top; 2 is better than 3 by far, crop as before, possibly a little tighter than before, try 25%; 5 is ok but probably doesn't illustrate so much more than the others on the page that I'd want to find space for it; 6 needs heavily cropping from all sides before I can even evaluate it; 7 has more detail in the body than 4 and tells me more about the wings, could do with a 20% crop from the right and is really very nice, be a shame not to use it somewhere. I'd put these up at about 1500x1000px to be of real value, and if you're getting weird results with colour profiles maybe the Lab would be a good idea, I'm not sure the GIMP handles profile conversion any better than WIRPT. Hope that's been some help. mikaultalk 05:33, 5 November 2009 (UTC)
-
Valley carpenter bee, Xylocopa varipuncta. Slightly clipped on top but in focus.
-
Valley carpenter bee, Xylocopa varipuncta. Not clipped off but slightly out-of-focus. which one to choose?
-
Acraea sp., Heliconiinae. Which pic is best?
-
Oxythyrea sp. Identify the flower. Crop needed? Is this photo needed? The article has much better close-ups.
- I don't know the answer, but I put them in a gallery for you so I thought I'd leave a message to let you know. They're beautiful, but I don't see much photography. Vimescarrot (talk) 21:08, 3 November 2009 (UTC)
- Thanks. I've edited some captions. Check out the pics in our bee article for some REALLY superb photography. This is rather second-rate in comparison. Zunaid 21:26, 3 November 2009 (UTC)
- The first one is a carpenter bee, Xylocopa sp.. The last one is Oxythyrea sp. (I'm going to write a stub article for that one soon). The butterfly species name eludes me for a moment, but it will come back to me in a minute or two. --Dr Dima (talk) 23:14, 3 November 2009 (UTC)
- Done: Oxythyrea article stub is up. --Dr Dima (talk) 06:56, 4 November 2009 (UTC)
- The before-last one (middle one in the lower row) is also a chafer beetle, family Scarabaeidae; there is not enough resolution to make a more accurate identification. --Dr Dima (talk) 23:47, 3 November 2009 (UTC)
- The butterfly in pictures 4 and 5 is Acraea sp. (Heliconiinae). --Dr Dima (talk) 03:18, 4 November 2009 (UTC)
Thanks for the ID's so far Dr. Dima. Photographic critique and input needed. A lot of the linked articles already have sufficient (and better IMHO) photos. Are these pics really then needed? Is there any other noticeboard I can spam for photographic input? Zunaid 10:51, 4 November 2009 (UTC)
- The second and third picture, the yellow carpenter bee, is most likely a male Xylocopa varipuncta, commonly called the Valley carpenter bee. Bugboy52.4 | =-= 01:08, 5 November 2009 (UTC)
Thanks. Do our articles really need these pictures? Especially when compared to those already in the articles? Should I upload high res versions? Zunaid 04:55, 5 November 2009 (UTC)
- If in doubt, you can at least publish them to EOL. They have a group on Flickr where you can upload images and tag them appropriately to have the system add your photo to the encyclopedia. I've submitted several myself, some of which are the only photos there of the species. Bob the Wikipedian (talk • contribs) 17:44, 5 November 2009 (UTC)
vision acuity over 20/400
Is this possible to have vision over 20/400. Do anybody have like 20/1000 or 20/700. From corneal scar I have had my vision was 20/400--209.129.85.4 (talk) 20:56, 3 November 2009 (UTC)
- This article [7] implies that such Snellen fractions do exist. --Cookatoo.ergo.ZooM (talk) 21:14, 3 November 2009 (UTC)
- Well, only being able to detect light and dark as if your eyes were closed could be described as 20/infinity. --Tango (talk) 22:15, 3 November 2009 (UTC)
- If you consider what those two numbers mean, it's clear: 20/100 (for example) would mean a person who is only able to read letters that a normally-sighted person can read at 100 feet if they move to 20 feet away from them. For there to be 20/1000 vision, you'd have to be only just able to see letters at 20 feet that someone with normal vision could see at around two tenths of a mile! Normal vision allows you to recognise letters when they subtend only 1/12th of a degree. So for someone to have 20/1000 vision would mean that they'd only just be able to read letters about 18 inches tall from 20 feet away. SteveBaker (talk) 03:44, 4 November 2009 (UTC)
- It doesn't have to be letters. It's just about resolving an image, whatever that image may be. The page linked to says the common way to measure 20/1000 vision would by getting to person to count fingers, in this case at 8 feet distance (although you would just report "CF 8'" rather than converting it to 20/1000). I guess that means that someone with normal vision can distinguish fingers at 400 feet (which sounds about right to me). --Tango (talk) 03:56, 4 November 2009 (UTC)
- Isn't vision worse then 20/400 classified as legally blind anyways? Googlemeister (talk) 15:31, 4 November 2009 (UTC)
- Depends on jurisdiction, but about that. There is more to life than whether or not you can claim benefits, though. --Tango (talk) 17:32, 4 November 2009 (UTC)
- Isn't vision worse then 20/400 classified as legally blind anyways? Googlemeister (talk) 15:31, 4 November 2009 (UTC)
- It doesn't have to be letters. It's just about resolving an image, whatever that image may be. The page linked to says the common way to measure 20/1000 vision would by getting to person to count fingers, in this case at 8 feet distance (although you would just report "CF 8'" rather than converting it to 20/1000). I guess that means that someone with normal vision can distinguish fingers at 400 feet (which sounds about right to me). --Tango (talk) 03:56, 4 November 2009 (UTC)
Human - 23 chromosome pairs
What happens when one is born with more or less than 23 pairs? --Reticuli88 (talk) 21:39, 3 November 2009 (UTC)
- It will cause one or another disease. There are many, depending on which chromosome was duplicated/deleted. See Aneuploidy. Someguy1221 (talk) 21:43, 3 November 2009 (UTC)
- The result will be a genetic disorder, not a disease. DRosenbach (Talk | Contribs) 02:40, 4 November 2009 (UTC)
- Well, technically it will "kill" the potential life (well before birth approaches), and the (semi-)survivable diseases caused by the monosomy and trisomy Someguy refers to are the rare exceptions. ~ Amory (u • t • c) 22:34, 3 November 2009 (UTC)
- Indeed. Miscarriage is often caused by a fault in the genes. --Tango (talk) 22:42, 3 November 2009 (UTC)
- According to the disease link - "A disease is any medical condition that impairs bodily function." Some genetic disorders will impair bodily function and will therefore be be diseases. In reality I'm struggling to think of a genetic disorder that would not impair bodily function. Richard Avery (talk) 08:38, 4 November 2009 (UTC)
- It wouldn't be a disorder if it didn't impair bodily function, it would be a neutral or beneficial mutation. Was anyone questioning the definition of disease? --Tango (talk) 17:42, 4 November 2009 (UTC)
- According to the disease link - "A disease is any medical condition that impairs bodily function." Some genetic disorders will impair bodily function and will therefore be be diseases. In reality I'm struggling to think of a genetic disorder that would not impair bodily function. Richard Avery (talk) 08:38, 4 November 2009 (UTC)
- Indeed. Miscarriage is often caused by a fault in the genes. --Tango (talk) 22:42, 3 November 2009 (UTC)
Biological distinction between Sickness and Contagiousness in Contagious diseases.
I understand that people can be contagious but not ill during the incubation stage of a disease. The opposite, being ill but not contagious, is commonly said to exist at the tail end of a disease. What is going on biologically during both of these periods? I'm starting to think that latter is a bit of folk science invented by ill people who are feeling cooped up and better enough to want to rejoin the world. -Craig Pemberton (talk) 23:12, 3 November 2009 (UTC)
- Certainly not. Infections are only contagious if they can be spread from person to person either directly or indirectly. If infectious particles exist in bodily fluids like sputum, semen and feces, spread can occur when shaking hands with an infected individual allows for a closed fecal-oral route of transit. That's great for transfer of things like E. coli and Hepatitis A. But certain infectious organisms do not congregate in fluids -- they may prefer to hide in other places (if not entirely, then at least for some portion of their infectious existence) or take on a noninfectious form when they are in certain fluid. Syphilis, for example, has three clinical phases -- patients with secondary syphilis are the most infectious, even though the infectious spirochetes are present from the time of inoculation. DRosenbach (Talk | Contribs) 02:53, 4 November 2009 (UTC)
- But why or how would an organism become noninfective. It would essentially be committing suicide if it did so willingly. -Craig Pemberton (talk) 03:57, 4 November 2009 (UTC)
- A pathogen is incapable of "becoming noninfective... willingly", as neither bacteria nor viruses have a free will. Rather, the outcome is decided by a competition between the pathogen and the immune system of the host. A viral or bacterial infection only rarely results in the death of the host. (That depends on the species of the pathogen and on the immune response of the host, of course). In most cases, pathogen is completely neutralized by the immune system of the host; at this stage the host no longer emits the pathogen. In some cases pathogen survives, and the infection becomes chronic and/or asymptomatic. In those cases the host remains a carrier of the pathogen, and may transmit it to others. --Dr Dima (talk) 05:41, 4 November 2009 (UTC)
- Of course free will is an illusion, but people use it all the time. What I meant was that if information in it's own genome shut it down, why? And if information in the human genome shut it down, how? And if the disease is inactivated then why are you still considered sick? This is what I have now: the human immune system becomes able to neutralize the germs and quickly does so. The person still shows symptoms because it takes a while to repair the damage. -Craig Pemberton (talk) 16:47, 4 November 2009 (UTC)
- A pathogen is incapable of "becoming noninfective... willingly", as neither bacteria nor viruses have a free will. Rather, the outcome is decided by a competition between the pathogen and the immune system of the host. A viral or bacterial infection only rarely results in the death of the host. (That depends on the species of the pathogen and on the immune response of the host, of course). In most cases, pathogen is completely neutralized by the immune system of the host; at this stage the host no longer emits the pathogen. In some cases pathogen survives, and the infection becomes chronic and/or asymptomatic. In those cases the host remains a carrier of the pathogen, and may transmit it to others. --Dr Dima (talk) 05:41, 4 November 2009 (UTC)
- But why or how would an organism become noninfective. It would essentially be committing suicide if it did so willingly. -Craig Pemberton (talk) 03:57, 4 November 2009 (UTC)
- It may be helpful to remember that the feeling you get of "being sick" is not entirely caused directly by the pathogen, but also includes your body's response to that pathogen (or, in the case of allergies, the body's response to something mundane). For example, fever often accompanies an infection such as the influenza, but many respiratory viruses prefer (or require) slightly cooler areas to do their dirty work. What's going on? Your body is raising its core temperature to help the fight against the infection. At other times, your body will react in a way that also suits the virus. For example, a runny nose helps flush the virus from your sinuses, but also aids the virus in spreading by releasing infectious material. But your incredibly complicated immune system sometimes runs rampant and sometimes simply keeps running after it no longer needs to. So, while your nose may be running as part of your body's attempt to flush your sinuses, the virus may now be long gone. This is apparently quite common when it comes to cough (OR alert) - coughing irritates your windpipe, which leads to... coughing... which irritates your windpipe. Your infection may be dead and gone for weeks and yet you keep on coughing because your throat is so badly worn out. I've had that situation (as diagnosed by a doctor): stop the cough by any means necessary for even a day or so and the rest of the problem just collapses. So, I was hacking like a hound-dog for a month but was completely non-infectious. Matt Deres (talk) 17:55, 4 November 2009 (UTC)
If "Big bang" is accurate...
If the universe's big bang beginning is accurate, then at that time all matter was together. We are also told that the light from distant galaxies hasn't made it all the way across the universe yet. I imagine that there is lots of relativity and stuff involved, but surely for both statements to be true some matter needs to have moved at faster than the speed of light. What am I misunderstanding? -- SGBailey (talk) 23:36, 3 November 2009 (UTC)
- Actually, we can see almost all the way to the big bang itself. Just a short while after the big bang, the universe became clear and light could start traveling. We can see back to this wall and it is called the cosmic microwave background radiation. But events which happened too recently for light from them to reach you, you cannot see yet. Even when you pour yourself a cup of coffee you can't observe it for a time = armlength/C. -Craig Pemberton (talk) 23:46, 3 November 2009 (UTC)
- It's generally assumed that everything in the universe came from one place, because it's hard to see how it could be so homogeneous otherwise. So it's all in our past light cone. But we can't see everything. The picture is something like this:
* /\ ^
@ / \ |
/ \ |
################## time
- We're at the top center and #### is the primordial fireball (the source of the cosmic microwave background). We can't see the event marked *, but we can see the matter that later does *. We can't see the event marked @ and we also can't see the matter that later does @, because the universe is opaque below the ####. But in principle if you extend the diagonal lines through the fireball they encompass the whole universe. The "edge of the visible universe" is defined by where the light cone hits the fireball. -- BenRG (talk) 00:51, 4 November 2009 (UTC)
- We need to be careful with our terminology. You are using "universe" to mean "observable universe" (which means everything that can be theoretically observed, not just things that can be observed using EM radiation). Anything outside the observable universe can't have any impact on us, so in a sense we can just say it doesn't exist (which is why people do often abbreviate the observable universe to just "universe"), but in another sense it is very likely that it does (the size of the universe happening to exactly correspond with the size of the observable universe would be a massive coincidence unless there is something going on that we don't know about - alternatively, the universe could be smaller than the observable universe, in which case we should be able to see the same objects multiple times as the light goes all the way around the universe). --Tango (talk) 01:00, 4 November 2009 (UTC)
- We're at the top center and #### is the primordial fireball (the source of the cosmic microwave background). We can't see the event marked *, but we can see the matter that later does *. We can't see the event marked @ and we also can't see the matter that later does @, because the universe is opaque below the ####. But in principle if you extend the diagonal lines through the fireball they encompass the whole universe. The "edge of the visible universe" is defined by where the light cone hits the fireball. -- BenRG (talk) 00:51, 4 November 2009 (UTC)
- The expansion of the universe isn't matter moving outwards through space, it is the space itself expanding. There is no speed of light limit on the expansion of space, just on matter moving through space. See Metric expansion of space. The standard analogy is blowing up a balloon with dots on it. The dots are stationary compared to the bit of balloon they are on, but they move apart from each other as the balloon expands. The further apart they are, the faster they move apart. For objects far enough away from us, they are moving away from us (or, rather, the distance between them and us in increasing - they aren't actually moving, that's the point) faster than the speed of light. At the moment, the relevant distance is about 45 billion light years, I believe. --Tango (talk) 00:07, 4 November 2009 (UTC)
- Many processes in physics are reversible. If no rules of physics are violated when "space" expands, then could it similarly contract without violating any law of physics? Hypothetically speaking, could the space between planet A and planet B (initially 10 light years) contract to .1 light year, making a trip between them much more convenient, so that it could be completed at .01 c in 10 years? By definition, there would be no faster than light travel. If we cannot explain why space expanded, can we set limits on the geometry of any future expansion (or useful contraction)? Edison (talk) 04:26, 4 November 2009 (UTC)
- Yes, metric expansion can run in reverse. It's not expected to ever happen in our universe, but a Big Crunch was once hypothesised as a possible end of the universe. I don't think it would make interstellar travel easier, though - if the universe contracted far enough to make a different within a single galaxy then I think it would pretty quickly end up destroying any habitable systems. --Tango (talk) 17:50, 4 November 2009 (UTC)
- Many processes in physics are reversible. If no rules of physics are violated when "space" expands, then could it similarly contract without violating any law of physics? Hypothetically speaking, could the space between planet A and planet B (initially 10 light years) contract to .1 light year, making a trip between them much more convenient, so that it could be completed at .01 c in 10 years? By definition, there would be no faster than light travel. If we cannot explain why space expanded, can we set limits on the geometry of any future expansion (or useful contraction)? Edison (talk) 04:26, 4 November 2009 (UTC)
- Question - Metric expansion of space says there are two reasons for expansion: inertia and a repulsive force. Inertia is a property of matter and forces act on matter so it sounds like expansion is matter just moving apart. I understand (from reading this desk) that the velocity at which an object moves away from us can be more than c because time (in that measure of velocity) is based on a clock moving with the object which slows down as it moves. My question is: how do the two reasons above "fit" with space itself expanding? Zain Ebrahim (talk) 08:15, 4 November 2009 (UTC)
- I'm not entirely sure about this bit (BenRG has tried to explain it to me, but I've never been entirely convinced). Even excluding the cosmological constant (which models that repulsive force you mention), the expansion isn't just inertia as we would usually consider it. It is caused by inertia, but that inertia causes the space itself to expand, that allows greater than light speed expansion. --Tango (talk) 17:50, 4 November 2009 (UTC)
- Question - Metric expansion of space says there are two reasons for expansion: inertia and a repulsive force. Inertia is a property of matter and forces act on matter so it sounds like expansion is matter just moving apart. I understand (from reading this desk) that the velocity at which an object moves away from us can be more than c because time (in that measure of velocity) is based on a clock moving with the object which slows down as it moves. My question is: how do the two reasons above "fit" with space itself expanding? Zain Ebrahim (talk) 08:15, 4 November 2009 (UTC)
- Note that in the balloon example, inertia of the balloon surface itself (or tiny little pieces of lead fixed to the surface, if that's an easier analogy) would pull the balloon further apart, and even in a way that the distance between any two pieces on the surface would grow faster than the distance between the same two pieces in 3-D space. --Stephan Schulz (talk) 18:08, 4 November 2009 (UTC)
- Can the "balloon" have "outward" "inertia"? The "outward" direction isn't really a direction at all from the perspective of someone on the balloon. I don't have a good understanding of this topic, but this seems very strange to me. Rckrone (talk) 23:44, 4 November 2009 (UTC)
- The balloon, yes, certainly. Matter in the real universe - I don't know, but I suspect so. Otherwise, the interia vs. gravity argument makes no sense. --Stephan Schulz (talk) 13:11, 5 November 2009 (UTC)
- Can the "balloon" have "outward" "inertia"? The "outward" direction isn't really a direction at all from the perspective of someone on the balloon. I don't have a good understanding of this topic, but this seems very strange to me. Rckrone (talk) 23:44, 4 November 2009 (UTC)
- Note that in the balloon example, inertia of the balloon surface itself (or tiny little pieces of lead fixed to the surface, if that's an easier analogy) would pull the balloon further apart, and even in a way that the distance between any two pieces on the surface would grow faster than the distance between the same two pieces in 3-D space. --Stephan Schulz (talk) 18:08, 4 November 2009 (UTC)
- Scratches head... Um, a quick break for a WP:OR or WP:SYNTH might be helpful! I don't particularly think Wikipedia is for writing essays on theoreticals like this. Don't make me insist you all find citations! This isn't a defined talk page so I think that means I can request them :) ♪ daTheisen(talk) 16:01, 4 November 2009 (UTC)
- Our priority here is answering the OP's question in a way they can understand. If linking them to a reference they can read for themselves is the best way to do that, we'll do it. If writing out a detailed answer ourselves is better, we do that. Finding citations isn't really necessary unless there is a dispute. --Tango (talk) 17:50, 4 November 2009 (UTC)
Thanks all. I don't think I understand it as well as I want yet, but I understand it more than I did. -- SGBailey (talk) 11:44, 5 November 2009 (UTC)
November 4
Spiritual Science. Rectifying Hypotheses
can i create my own hyperlink-web here of my findings and studies with this 'spirituality'? i believe people would be interested in these things. i simply would like to be recognized as the author. please tell me my options. i simply want this question answered, i will not waste my time. —Preceding unsigned comment added by Love me alway (talk • contribs) 03:55, 4 November 2009 (UTC)
- Sorry, but Wikipedia is not a venue for original research. — Lomn 04:05, 4 November 2009 (UTC)
- Such a compilation may be acceptable on your user page or in a user-space sub-page. See the Help for User Subpages and guidelines for allowable subpages. Again, Wikipedia is not the place to conduct original research; but if you are merely compiling and organizing links to other Wikipedia articles that you find useful, you can put it in your user space. This area has a little less regulation for content, as it is not technically part of the encyclopedia. As far as ownership, all content must be submitted under the Creative Commons and GFDL licenses - keep this in mind. You do not own your user-page or the content you submit to it. Finally, remember that even though Wikipedia offers you some freedom in the user-page space, Wikipedia is not your web host. Our goal is to write an encyclopedia; your user-page space is really supposed to help you (and others) to make contributions to actual encyclopedia articles. Nimur (talk) 05:58, 4 November 2009 (UTC)
- Submitting material to Wikipedia is unwise if you wish to be recognized as the author. You could easily be accused later of copying (your own text) from Wikipedia. Cuddlyable3 (talk) 16:16, 4 November 2009 (UTC)
- You can generally prove that you were the author of a particular thing by examining the "history" of the item in question. However, there is nothing whatever you can do to prevent someone from changing what you wrote - or copying it - or deleting it - or anything else. The open licensing of Wikipedia not only allows that - it actively encourages it. However, your own "findings" about something are completely unwelcome here. The goal is for all information to be generated in a neutral manner by reference to respected source material. Nothing you think up for yourself is allowed into any Wikipedia article...so what you suggest would likely be vehemently opposed from all sides! SteveBaker (talk) 22:13, 4 November 2009 (UTC)
- I interpreted "findings" to mean "things the OP found on the web and wants to link to." If the "findings" are actually synthesis of original research, then as Steve has pointed out, they should not be published on Wikipedia. We have a strong policy against publishing original research here. Nimur (talk) 17:44, 5 November 2009 (UTC)
Name reactions in Chinese Wikipedia
Look at the articles about name reactions in Chinese Wikipedia. Why don't they translate the names into Chinese? --128.232.251.242 (talk) 10:37, 4 November 2009 (UTC)
- Chinese is in general iconic rather than sound based. The problem is with us using Chinese designations as they'd see it is us not using the proper icon but just a particular sound. Dmcq (talk) 10:54, 4 November 2009 (UTC)
- But almost all the people do have a Chinese name, with almost no exceptions, for instance see zh:Category:Living people. Adolph Wilhelm Hermann Kolbe translates to zh:阿道夫·威廉·赫尔曼·科尔贝 in Chinese. So why don't they translate the name Kolbe in Kolbe electrolysis into Chinese? --128.232.251.242 (talk) 11:12, 4 November 2009 (UTC)
- I didn't see any specific guideline there about it. I guess their chemists must just find it easier. You could always ask on a talk page there. Dmcq (talk) 12:33, 4 November 2009 (UTC)
- They don't translate the name because it's a proper noun specifically referring to the reaction. If it were also translated it would be a generic adjective of something. It looks odd because of the huge contrast between roman characters, but it's standard convention in most types of math and science to hold the names. Just because it was able to translate "Kolbe" doesn't mean it came out as "Kolbe"; in its eyes it translated "kolbe", which is a very important distinction. This all happens a whole lot in Japanese, too. Why don't we use their characters for their historical persons or theorems? It's "mostly" easier to move into a lettered format than from that into complex characters. I don't know the term of it in traditional Chinese, but it's "Romanji" in Japanese, literally the romanization of the real language so that most anyone in the world can try to pronounce a word. A nod to European influence via early trading routes into the long-established Eastern cultures. ♪ daTheisen(talk) 15:53, 4 November 2009 (UTC)
- I think the reason is really because people are lazy and there are many different (conflicting) complex standards for transliterating foreign names. PRC, Hong Kong and Taiwan all have different transliteration schemes with different characters, which editors would need to deal with using templates, which has quite a learning curve. It's nowhere near as simple as romaji for Japanese, and it's further compounded by the great firewall of China (although I heard Wikipedia's been unblocked?). --antilivedT | C | G 06:17, 5 November 2009 (UTC)
- They don't translate the name because it's a proper noun specifically referring to the reaction. If it were also translated it would be a generic adjective of something. It looks odd because of the huge contrast between roman characters, but it's standard convention in most types of math and science to hold the names. Just because it was able to translate "Kolbe" doesn't mean it came out as "Kolbe"; in its eyes it translated "kolbe", which is a very important distinction. This all happens a whole lot in Japanese, too. Why don't we use their characters for their historical persons or theorems? It's "mostly" easier to move into a lettered format than from that into complex characters. I don't know the term of it in traditional Chinese, but it's "Romanji" in Japanese, literally the romanization of the real language so that most anyone in the world can try to pronounce a word. A nod to European influence via early trading routes into the long-established Eastern cultures. ♪ daTheisen(talk) 15:53, 4 November 2009 (UTC)
- I didn't see any specific guideline there about it. I guess their chemists must just find it easier. You could always ask on a talk page there. Dmcq (talk) 12:33, 4 November 2009 (UTC)
- But almost all the people do have a Chinese name, with almost no exceptions, for instance see zh:Category:Living people. Adolph Wilhelm Hermann Kolbe translates to zh:阿道夫·威廉·赫尔曼·科尔贝 in Chinese. So why don't they translate the name Kolbe in Kolbe electrolysis into Chinese? --128.232.251.242 (talk) 11:12, 4 November 2009 (UTC)
- In fact our own languages reference desk would probably be best at WP:RD/L. My guess is that people read and write about chemistry but names of people and places are things one says. Even so you will quite often see some special name translated by meaning rather than sound into English. Dmcq (talk) 15:42, 4 November 2009 (UTC)
It's a myth that Chinese is iconic rather than sound-based. All languages are phonetic, but not all languages have a phonemic writing system. John Riemann Soong (talk) 16:39, 4 November 2009 (UTC)
- All spoken languages are phonetic. Sign languages are not, and it is possible to have an entirely written language which is not simply a representation of a spoken language. In fact, I gather written literary Chinese (Literary Sinitic) is quite close to this [8]. 86.142.224.71 (talk) 18:38, 4 November 2009 (UTC)
- Which is what I meant. It can be pronounced very differently in different dialects. Dmcq (talk) 22:27, 4 November 2009 (UTC)
- Not only pronounced differently: it is a different language to most (all?) of the 'dialects'. Children who have a mother tongue other than Mandarin (and even those who have Mandarin?) have to learn it in order to write. 86.142.224.71 (talk) 22:37, 4 November 2009 (UTC)
- Classical Chinese may be helpful here Nil Einne (talk) 10:01, 5 November 2009 (UTC)
- Excellent. And it links to Vernacular Chinese, which is the one that children have to learn Mandarin to be able to read. 86.142.224.71 (talk) 16:28, 5 November 2009 (UTC)
- Classical Chinese may be helpful here Nil Einne (talk) 10:01, 5 November 2009 (UTC)
- Not only pronounced differently: it is a different language to most (all?) of the 'dialects'. Children who have a mother tongue other than Mandarin (and even those who have Mandarin?) have to learn it in order to write. 86.142.224.71 (talk) 22:37, 4 November 2009 (UTC)
- Which is what I meant. It can be pronounced very differently in different dialects. Dmcq (talk) 22:27, 4 November 2009 (UTC)
universities
can i known what are the top universities in uk for m pharmacy —Preceding unsigned comment added by Nagtej (talk • contribs) 13:39, 4 November 2009 (UTC)
- Sorry, I'm not sure how I can help. The same way that Wikipedia isn't a source or original research, we also can't try to create new ideas using information from Wikipedia. We hold to a standard of neutral point of view in articles, so there would be no way to to make guesses like that even if we thought we should! Good luck... ♪ daTheisen(talk) 15:57, 4 November 2009 (UTC)
- The QUB School of Pharmacy has been rated as the top Pharmacy School in the UK in the 'Times Good University Guide 2010' [www.qub.ac.uk/schools/SchoolofPharmacy/dl/] and The University of Nottingham in the 2006 Times Good University Guide [www.nottingham.ac.uk/pharmacy/undergraduates/index.php]. Tracking down the mentioned guide will surely lead to others. 75.41.110.200 (talk) 16:13, 4 November 2009 (UTC)
- UCAS (Universities & Colleges Admissions Service) can help you with information on undergraduate degree programmes at UK universities and colleges.Cuddlyable3 (talk) 16:10, 4 November 2009 (UTC)
- Yet another best of list at the guardian. --Tagishsimon (talk) 16:14, 4 November 2009 (UTC)
- UCAS (Universities & Colleges Admissions Service) can help you with information on undergraduate degree programmes at UK universities and colleges.Cuddlyable3 (talk) 16:10, 4 November 2009 (UTC)
- The QUB School of Pharmacy has been rated as the top Pharmacy School in the UK in the 'Times Good University Guide 2010' [www.qub.ac.uk/schools/SchoolofPharmacy/dl/] and The University of Nottingham in the 2006 Times Good University Guide [www.nottingham.ac.uk/pharmacy/undergraduates/index.php]. Tracking down the mentioned guide will surely lead to others. 75.41.110.200 (talk) 16:13, 4 November 2009 (UTC)
- Sorry, I'm not sure how I can help. The same way that Wikipedia isn't a source or original research, we also can't try to create new ideas using information from Wikipedia. We hold to a standard of neutral point of view in articles, so there would be no way to to make guesses like that even if we thought we should! Good luck... ♪ daTheisen(talk) 15:57, 4 November 2009 (UTC)
- See here for the Times Good University Guide for Pharmacology and Pharmacy. --Tango (talk) 17:53, 4 November 2009 (UTC)
Was the discovery of evolution "inevitable" in the 19th Century? Why
I visited an aquarium last week, and one of the exhibits said that because of changes in scientific thinking the discovery of evolution was "inevitable" in the 19th Century. It mentioned that Charles Darwin and Alfred Wallace discovered it independently, and said that even if they had not seen it the theory's time had come and someone else would have done soon. Is this true, or if it was not for these two discoverers could we still not understand evolution today? If it is true what changes in thinking and previous discoveries made the theory of evolution inevitable? As a side question do you "discover", "invent", or "author" a theory - I don't know quite what to write! -- Q Chris (talk) 15:01, 4 November 2009 (UTC)
- It's hard/impossible to speculate whether anything that already happened was "inevitable" but I'm fairly certain that someone else would have struck upon the idea before too long. A lot of the original theory was essentially "Wait a minute... That animal looks a lot like that other animal" and you don't need a rich guy or a traveler to notice that. The Great man theory is probably relevant here, but essentially it's not really possible to factually answer your question. ~ Amory (u • t • c) 15:14, 4 November 2009 (UTC)
- Oh, and as for your last question, you can say "constructed" or "formulated." ~ Amory (u • t • c) 15:16, 4 November 2009 (UTC)
- It was inevitable if you consider that genetic studies were independent of evolution. While the early studies didn't know anything about genes or DNA, they were tracing patterns of inheritance from parent to child, such as colors of kernels of corn and eye color in mice. Eventually, someone would have to recognize that traits were being passed from parent to child. As soon as someone were to stumble upon mutation, evolution would be evident. -- kainaw™ 15:20, 4 November 2009 (UTC)
- The idea of evolution generally was well-known and well-discussed well before Darwin and Wallace. The idea of natural selection being the mechanism of evolution probably would have come out at some point if both of those two had been hit by a train before their time. This distinction is rather important. It is not a matter of saying "this animal looks like this one"—people had already been doing that for a long time, even in the scientific sphere (see, e.g. Erasmus Darwin, Jean-Baptiste Lamarck, and less scientifically, Robert Chambers, etc.).
- There were a lot of people thinking along similar lines at the time—it was definitely "in the air". Darwin is especially well-known because he articulated it in a rather careful form, with lots of evidence, and with all the power of his already-established scientific name attached to it. (Wallace was, in this sense, very much at a disadvantage.) He had powerful friends who assured that the theory would be taken seriously and given attention within the scientific community and not dismissed as just a variation on Lamarck or as just political claptrap (Cf. Vestiges of the Natural History of Creation).
- As for the distinction between "discoveries", "inventions", "authors"—it's a great question, one that professional historians have actually argued quite a lot about. It depends on your conception of authorship itself, and on your conception of what is to be authored. Are theories hanging out there "in the world", waiting to be discovered? Or is the process of articulating a theory a creative act as well as an objective one? I tend to think the answer is somewhere in between—there is a "core" reality to be "discovered", but the aspects of it that get written about, and the ways in which they are formulated in human language, and made convincing and compelling, are a definitely parts of an authorial intervention—an "invention", one could say. This is much easier to see as time goes on—Darwin's theory of natural selection, as he articulated it, contains quite a bit of "nature" in it, but it is very much a work of the particular man himself, and his formulation of it, his preoccupations, his way of arguing it, all reflect that very strongly. --Mr.98 (talk) 15:28, 4 November 2009 (UTC)
- (ec, and now mostly redundant. But I wrote it, so you better read it ;-): "Evolution" was evident quite a while before Darwin and Wallace from looking at fossils. See history of evolutionary thought. Erasmus Darwin described evolution in the late 19th century, and Lamarck formulated his idea of species evolving to better meet environmental conditions in 1809. What Darwin (and Wallace) added was the mechanism of natural selection working on variations of inherited traits, not the idea of evolution itself. --Stephan Schulz (talk) 15:32, 4 November 2009 (UTC)
- I would argue that yes, evolution was an inevitable 'discovery'. It's extremely elementary that weak things are removed over time and robust things remain. It applies to everything across the spectrum, from the erosion of mountains, to biological features, to ruling powers. I suppose it appeared when it did as rationality was starting to question the religious hegemony of past centuries, not because the idea itself was necessarily of profound importance. Vranak (talk) 16:09, 4 November 2009 (UTC)
- I think you underestimate the difficulty of making the scientific case (ignore the religious stuff for a moment—it mattered for some, not for others). Remember that when Darwin proposed evolution there was no good model for biological heredity at all, a relatively new knowledge of the fossil record, and no consensus over the age of the Earth. This makes making a compelling scientific argument about natural selection rather difficult. If the rate of change is too slow, and the Earth is too young, then it doesn't work. Without a model of heredity that allows for things like mutations, you don't have any way for speciation to take place. These are non-trivial concerns. There were plenty of people before Darwin who waived their hands and said "oh this is a common principle to all things so it applies to humans" (again, see Robert Chambers), but 1. they were more often than not wrong (because there are a lot of candidate "common principles"), and 2. they were totally uncompelling (because just because something works in one arena doesn't mean it works in another). Even in Darwin's case, it was not totally compelling—most scientists did not accept natural selection as the mechanism of evolution until long after he had died and the modern evolutionary synthesis was developed (some 70 years after Origin of Species!). (And this latter point has nothing to do with religion—they accepted evolution in general.) --Mr.98 (talk) 16:32, 4 November 2009 (UTC)
- If you read The Origin of Species it's quite clear that Darwin wasn't proposing an original idea. Rather he did a lot of careful synthesis and research in order to champion an idea. The Origin gave a confused set of ideas clarity and made it obvious how and why they had to be true. I think you can divide scientific "discoveries" into three kinds;
- "wtf is this" discoveries, finding something, such as a fossil or a strange unexpected residue, eg Ardi or teflon
- "eureka" discoveries, a sudden stroke of genius like the Dirac equation and Archimedes' principle
- "synthesis" "discoveries" like the theory of evolution or plate tectonics. These are the best and hardest.
- In the popular imagination, people think science is largely of the "eureka" type, which are actually probably the rarest. -Craig Pemberton (talk) 16:45, 4 November 2009 (UTC)
- I like that classification, and I will make the phrase "wtf discoveries" part of my working vocabulary ;-) --Stephan Schulz (talk) 18:10, 4 November 2009 (UTC)
self-ionisation of glacial acetic acid
How does it compare to water? How does the entropy contribution of the reaction change? What about enthalpy of self-ionisation? John Riemann Soong (talk) 16:37, 4 November 2009 (UTC)
Current and time threshold
I'm trying to design a circuit that activates a transistor when a photodiode has been illuminated at a certain intensity for a certain time, but only when at least a certain current is produced non-stop during that time, e.g. 5µA for 50ms. Essentially I want it to start a "stopwatch" the instant the current rises above 5µA, and reset that stopwatch the instant the current drops below 5µA. If the timer reaches 50ms before it is reset, a monostable LMC555 fires and holds a transistor (logic-level MOSFET or Darlington BJT - I haven't decided yet) high for about half a second. Ideally the entire procedure would be analogue, since I want to keep everything small and simple, and I also want it to use as little power as possible so I can run it off a minimal battery like a button cell. I'd also like to keep it as small as possible physically.
I've brainstormed about this for a while, but I can't figure out any good, simple ways to do it. Any ideas? --Link (t•c•m) 16:40, 4 November 2009 (UTC)
- First you need to convert the photodiode current into a voltage with either a a current to voltage op amp circuit or just a resistor. The o/p of the I_V converter needs to go to some sort of Schmitt trigger circuit with its threshold set to the equivalent of your desired photodiode current. This will give you a high level out when the current is above threshold. Now you need to use the rising edge of that pulse to start your timer and the falling edge to stop your timer. For the timer circuit I would tend to go for a gated square wave oscillator and a counter, but there must be other ways. Trouble is all the circuitry described probably wouldnt run under 5v. Not sure if CMOS would work reliably at 1.5V--79.67.31.17 (talk) 18:31, 5 November 2009 (UTC)
 . Cuddlyable3 (talk) 20:50, 5 November 2009 (UTC)
. Cuddlyable3 (talk) 20:50, 5 November 2009 (UTC)
In the circuit shown the transistor conducts for 0.5s after the photodiode has been lit for 50mS and can conduct longer if the photodiode is lit longer. Cuddlyable3 (talk) 21:05, 5 November 2009 (UTC)
- Cheers! I'll check it out later (I'm not exactly awake yet). --Link (t•c•m) 09:22, 6 November 2009 (UTC)
- Looks like a nice simple circuit that should work well. However, I think the photo diode is shown connected the wrong way round in this circuit as it needs to be used in the photoconductive (not photovoltaic) mode. Also the OP wanted operation from a single cell (1.5v) which is far more complex unless a little inverter is used. —Preceding unsigned comment added by 79.75.83.17 (talk) 15:04, 7 November 2009 (UTC)
- I've actually decided to go about this a bit differently (using a microcontroller) since I need high noise immunity. I did notice the photodiode was connected the wrong way around. Also, I was actually planning to use a 3V button cell - there is very very little that happily runs off 1.5V. :) --Link (t•c•m) 17:40, 7 November 2009 (UTC)
reactivity esters v. carboxylic acids
Why are carboxylic acids classified as less reactive than esters? Alkoxides tend to be worse leaving groups than hydroxides, right? (Except methoxide, which has a lower pKa). Alkyl groups tend to be electron donating, right..? (Or do they also delocalise some of the negative charge on the ethoxy ester oxygen?) John Riemann Soong (talk) 16:51, 4 November 2009 (UTC)
- How do you mean "less reactive" anyways? In terms of cleaving the C-O bond? Hydroxide is a worse leaving group than any alkoxide, so clearly in terms of -COOR -> -CO + OR if R = alkyl is more favorable than R = H. In other ways; for example in terms of reactivity with bases, carboxylic acids are more reactive. You need to define what reaction you are trying to do! --Jayron32 21:12, 4 November 2009 (UTC)
- Acyl substitution, naturally. (Acid-base reactions are trivial...) How is -OH a bad leaving group compared to an alkoxide? The pKa of water is 15.7; the pKa of most alcohols is 16-18. (Save phenols) John Riemann Soong (talk) 21:41, 4 November 2009 (UTC)
- Alkoxides are more stable ions because they are "softer"... in other words, there is greater dispersion of electric charge across a larger ion. That makes it more kinetically favorable; i.e. it sticks around longer. There's more going on here than just looking at the pKa, which is basically "H+ affinity" and not much else. Thermodynamically, it is harder to remove a proton from an alcohol than from water (and thus, conversely, it is more energetically favorable to protonate an alkoxide anion than to protonate hydroxide). So, if one considers the controlling factor in the reaction to be "Le Chatelier's principle" ONLY (that is, the protonation of the leaving group driving the equilibrium towards completion), then it would appear that hydroxide would make a better leaving group. However, there are other factors to consider beside that; for the equilibrium constant for the leaving group process (i.e. RCOOR <-> RCO+ + -OR). The more OR is made, the faster it will be quenched by availible H+ ions. The difference in the pKa's is probably not nearly as great as the difference in the K's for that process, for example if R = H vs. R = alkyl. You have a complex mix of processes here, and the kinetics of the slowest step is the driving force here. Protonation of the anion is a relatively fast step, so the difference in the pKa's is unlikely to be a major factor here. --Jayron32 05:58, 5 November 2009 (UTC)
fetus/mother sharing
When a pregnant women has an orgasm does the fetus experience pleasure or the orgasm as well? 71.100.13.177 (talk) 16:58, 4 November 2009 (UTC)
- The umbilical cord doesn't contain any nerves, so the fetus couldn't experience the orgasm itself. They might get some of the hormones that are released during orgasm (oxytocin, prolactin and maybe some others), which might give the fetus the same feelings of pleasure and relaxation following the orgasm. --Tango (talk) 17:59, 4 November 2009 (UTC)
- The main endogenous opioid associated with orgasm is β-endorphin, which can cross the placental barrier, so it seems plausible that the fetus would experience the opiate-high elements of orgasm, but that's a long way from the full and complex emotional and physical experience. In any case, I seriously doubt that the answer is known. --Sean 18:14, 4 November 2009 (UTC)
Antabus
In the film Skavabölen pojat, the family's father, already becoming slightly alcoholic when his sons are in pre-school age, is shown having taken an Antabus pill to try to cure his alcoholism. He shows his sons his bare stomach, where this Antabus pill has supposedly lodged itself firmly enough to be outwardly visible and tangible. A decade later, desperate to drink more alcohol, he is shown to surgically remove this pill from his stomach, so he can continue drinking without having any nauseous effects. Now I have never had to take Antabus myself, and I hope I never will. So therefore my question is out of scientific curiosity: The film gives the impression that once an Antabus pill is taken, it permanently lodges itself in the person's stomach, never dissolving. This is entirely unlike I have come to understand pills work - they should dissolve within days. How is this? How do Antabus pills work? Are they really permanent or have I just misunderstood the film's hints? JIP | Talk 19:59, 4 November 2009 (UTC)
- The Disulfiram article has information about the pharmacology of Antabuse. The effect is most certainly not permanent. The film appears to have taken some liberties with the mechanism of action. --- Medical geneticist (talk) 20:26, 4 November 2009 (UTC)
- According to our article, the half life of Antibus (disulfiram) is somewhere around 60-120 hours, and it can have some effect for up to two weeks. Red Act (talk) 20:30, 4 November 2009 (UTC)
Opting out of evolution in biology classes
Currently, many high-school biology classes allow parents to opt their students out of dissections if they are morally or religiously opposed to them. Why isn't a similar system put in place for the teaching of evolution? --J4\/4 <talk> 20:24, 4 November 2009 (UTC)
- Is studying evolution banned by any religion? I'm fairly sure actual dissection is banned by the religion itself, not just the parents. Vimescarrot (talk) 20:30, 4 November 2009 (UTC)
- In some schools, it is. In my California public high school in the 1990s, you could opt out of the evolution unit if your parents wanted you to. You had to go sit in study hall for those two weeks, or whatever length of time it was, while the rest of us stared at pictures of horses' feet. I'm not sure if anyone in my class did it; if it did, it was only one or two. (It was a very boring unit, incidentally—not nearly as racy as they had let on—but not as boring as being in study hall, probably.) I imagine that this issue, like all U.S. school-curricula issues, varies not only state by state, but probably even school district by school district. --Mr.98 (talk) 20:31, 4 November 2009 (UTC)
- There is in the UK. I once helped out on a school trip that involved a visit to a natural history museum and we had to be careful what we said because one of the children wasn't allowed to learn about evolution (there was only one exhibit that we had to gloss over, the rest was pretty safe). --Tango (talk) 20:32, 4 November 2009 (UTC)
- Schools are flexible, and should be. As a seven year old in the UK I adamantly came out as an atheist and refused to take part in anything related to Christmas or nativity because I said it was religious propaganda. I recall I was spared learning Carols and poems about Jesus and given parts of Old Possums book of cats to memorise instead. I doubt the school even bothered to check with my parents, and fifteen months later I softened and agreed to play Noah in a play (which didn't bother me because no one actually believes in Noah). I guess in an ideal world children should be able to opt themselves out of anything as soon as they know enough about it to feel they could make a decision. But I am far from sure that parents should be allowed to opt their children out of things, especially not for bad reasons. To really put the cat amongst the pigeons my reading of this as a Christian is that Genesis clearly explains "knowledge of Good and Evil corrupts" which is why I don't want my own children to see explicit violence or nasty but there is no forbidden fruit on a tree of "knowledge of blindingly obvious consensus science" --BozMo talk 20:42, 4 November 2009 (UTC)
- IMO all teaching should be done in a neutral manner so there should be no need for anyone to opt out. Knowledge is always good, it is the application of knowledge that can be bad. --Tango (talk) 20:50, 4 November 2009 (UTC)
- Not clear what "knowledge" is then, and when it demerges from experience. "Knowledge of how extreme pain feels"? even if you do disagree with Einstein about weapon technology... --BozMo talk 21:04, 4 November 2009 (UTC)
- IMO all teaching should be done in a neutral manner so there should be no need for anyone to opt out. Knowledge is always good, it is the application of knowledge that can be bad. --Tango (talk) 20:50, 4 November 2009 (UTC)
- Schools are flexible, and should be. As a seven year old in the UK I adamantly came out as an atheist and refused to take part in anything related to Christmas or nativity because I said it was religious propaganda. I recall I was spared learning Carols and poems about Jesus and given parts of Old Possums book of cats to memorise instead. I doubt the school even bothered to check with my parents, and fifteen months later I softened and agreed to play Noah in a play (which didn't bother me because no one actually believes in Noah). I guess in an ideal world children should be able to opt themselves out of anything as soon as they know enough about it to feel they could make a decision. But I am far from sure that parents should be allowed to opt their children out of things, especially not for bad reasons. To really put the cat amongst the pigeons my reading of this as a Christian is that Genesis clearly explains "knowledge of Good and Evil corrupts" which is why I don't want my own children to see explicit violence or nasty but there is no forbidden fruit on a tree of "knowledge of blindingly obvious consensus science" --BozMo talk 20:42, 4 November 2009 (UTC)
- Personally, I'd say that one can be a well educated adult without having engaged in dissections. It takes some effort to get the same insights from books and such, but it is certainly possible. On the other hand, I would say that one's education is grossly deficient if you don't understand the basic principles of evolution and the evidence for it. In my opinion, that's true even if one chooses not to accept evolution as factually true. It would be like not learning the atomic theory of matter, or not learning the structure of the solar system. Sure one can survive without that knowledge, but there is rather something incomplete with an education that skips over such basic issues. So, personally, I would resists efforts to allow students to opt-out of evolution discussions on the grounds that it really deprives the student of an important and basic science understanding. I would also point out that evolution is included on many standardized science tests, including those required for high school graduation in some areas. Dragons flight (talk) 20:52, 4 November 2009 (UTC)
- I kind of agree but does that mean I can also force them to learn Shakespeare or force them to learn to swim "one's education is grossly deficient"? I am just not sure I have the right to force it on people... --BozMo talk 21:00, 4 November 2009 (UTC)
- You can object to the whole principle of compulsory education if you want, but you won't find many people that agree with you (other than children that hate school!). --Tango (talk) 21:35, 4 November 2009 (UTC)
- No I am not amongst these folk Compulsory_education#Criticism but really we are talking compulsory syllabus, not compulsory education. You could drop biology 6 years younger than you were allowed to drop Latin at my school. How much evolution is really core education versus say Shakespeare? Pre genetics the careers advice used to be "if you can do maths and want to, do maths, if you can do maths and want to do science do Physics, if you cannot do maths and want to do science do Chemistry, if you cannot do science but want to do science do biology". Clearly I am outraged at having to do so much Latin, but I don't want biology to become the new Latin either... :) --BozMo talk 22:01, 4 November 2009 (UTC)
- Evolution is a more useful concept than Shakespeare. You can use it to find homologues, trace human migrations, do gene mapping, discover drugs, discover gene interactions, model ecological populations, disruptions and ecological balances. Plus, advanced evolution involves a lot of mathematical modelling not unlike that of say, finance. Evolution >> literature. John Riemann Soong (talk) 05:47, 6 November 2009 (UTC)
- No I am not amongst these folk Compulsory_education#Criticism but really we are talking compulsory syllabus, not compulsory education. You could drop biology 6 years younger than you were allowed to drop Latin at my school. How much evolution is really core education versus say Shakespeare? Pre genetics the careers advice used to be "if you can do maths and want to, do maths, if you can do maths and want to do science do Physics, if you cannot do maths and want to do science do Chemistry, if you cannot do science but want to do science do biology". Clearly I am outraged at having to do so much Latin, but I don't want biology to become the new Latin either... :) --BozMo talk 22:01, 4 November 2009 (UTC)
- You can object to the whole principle of compulsory education if you want, but you won't find many people that agree with you (other than children that hate school!). --Tango (talk) 21:35, 4 November 2009 (UTC)
- I kind of agree but does that mean I can also force them to learn Shakespeare or force them to learn to swim "one's education is grossly deficient"? I am just not sure I have the right to force it on people... --BozMo talk 21:00, 4 November 2009 (UTC)
I don't see how you could practically skip the evolution section. As somebody said, nothing in biology makes sense except in light of evolution -- what would you do for the rest of the course? Looie496 (talk) 21:50, 4 November 2009 (UTC)
- There are two counteracting principles here:
- Firstly, one cannot intelligently criticize something unless you've learned a fair bit about it first. Children of parents who disbelieve in evolution are precisely the ones who NEED to be taught it because they'll never learn it any other way - and they'll be incapable of making their own minds up about it as adults unless they were taught it while their minds were still flexible enough to absorb it. That cuts both ways - I don't think the children of atheists should be able to prevent their kids doing at least a basic comparative religion class. That there are religions is a fact - and understanding a reasonable range of them is well worth-while. So long as the class sticks to the observable facts (in both cases) - there should be no problem.
- However, we have a problem. Kids are only in school for so long - and their capacities for maintaining focus is somewhat limited. So we can't go in and teach them absolutely everything about everything. Some things have to take a higher priority. Fundamentals - math, literacy, language, basic science - all have to have a certain amount of time assigned to them, it's just unavoidable. The amount of time left in the school year after the essentials determines how much exposure to these other topics one gets. It's crucial that they see a little of everything that the world of knowledge has to offer - but spending (say) an entire year on evolution or on comparative religion is far too much.
- IMHO, parent's opinions should count for very little indeed. It's flat out not right that a child should be forcably prevented from learning things that their idiot parents failed to grasp. The entire reason that most countries in the world have mandatory education for children is because their parents can't be trusted to do the right thing otherwise. Being able to choose not to have their children learn evolution (or comparative religion) for a few weeks is no more defensible than allowing parents to not have their children be educated at all.
- If you allow parents to cherry-pick the courses they want their kids to attend - on religious grounds, or any other grounds for that matter - then pretty soon you're going to have parents who adhere to Sharia law deciding that their female children should not be educated at all past the age of 8. As a nation, we come to a consensus as to what needs to be taught - and everyone should be taught it.
- SteveBaker (talk) 22:02, 4 November 2009 (UTC)
- Apart from the "as a nation" bit which we are clearly not, I find it hard to disagree with any of this. Up until the child can make an informed refusal they should be taught everything. How much they get taught to make an informed refusal is subjective. --BozMo talk 22:05, 4 November 2009 (UTC)
- I'm not aware of any schools in the US that allow you to opt-out of dissection based on religious reasons. Given separation of church and state, it won't be an issue they can make lightly. Most schools either fail the students for that assignment, or make the assignment "free" so there is no grade for it. — The Hand That Feeds You:Bite 22:22, 4 November 2009 (UTC)
- The reason I mentioned it is because it is that way at my (public) school. If a student brought in a signed note from a parent or guardian explaining why dissections are against his/her moral or religious principals, the student would be given an alternate assignment instead. ----J4\/4 <talk> 23:10, 4 November 2009 (UTC)
- Where I grew up, one could get out of the 9th grade dissections for any reason whatsoever (not just religious ones). However, one wasn't allowed to opt-out of the dissections in the AP Bio class (typically 12th grade), but then the AP class was optional while the 9th grade class was mandatory. I don't recall what they did about grades in 9th grade, but I don't recall it being a big deal. Dragons flight (talk) 00:33, 5 November 2009 (UTC)
- Another question that this raises is, what other subjects could one opt out of? Sex education is the common one—again for moral reasons. Whether that is something that the parent should get to determine or not is a pretty push-button topic. What about select episodes in US history that one doesn't like the presentation of? Certain objectionable books? It's a rather nasty slippery slope to go down—which is why school boards usually set overall standards that are held to, rather that considering it on a case-by-case basis. In any case, there is no quick-and-easy answer, as it is not just a question about evolution, but about the rights of parents v. the rights of states, the goals of compulsory education, and so forth. --Mr.98 (talk) 23:16, 4 November 2009 (UTC)
- The objection against dissection runs deeper than simply "religion versus science" and I think it's useful to make note of that. Dissecting animals involving killing things. They're "just" animals, but let's not sugarcoat it. Living things are being killed so kids can look at them. Whether it stems from religious or personal morality, the concept that killing things - any things - is a "bad thing" is extremely ancient and cannot be lightly cast aside. Being able to poke around inside a dead animal to learn anatomy is arguably a good enough justification for doing it, but it's hardly a foregone conclusion - only a tiny percentage of the kids who partake in it will ever make any use of that knowledge ever again. Don't get me wrong; I did it and I had no qualms about it, but I'm honestly not sure how much I actually learned from it (that I didn't concurrently or later learn from models, videos, books, etc.) My later studies - which included studying human bodily remains - didn't hinge upon splitting open a frog and a worm in grade 10 Biology. Matt Deres (talk) 01:50, 5 November 2009 (UTC)
- But that's precisely the point. While 99% of students seemed to have gained nothing from it - and some of them were grossed-out, maybe had to run to the bathroom to puke, maybe just couldn't bring themselves to do it, maybe took that cue to become Vegans - the other 1% may have become so inspired by the process that they decided to become surgeons or to enter the field of anatomy, biology, zoology or whatever. The problem is that we don't know who those 1% are until we have them dissect something - and we need that early inspiration in order to get kids to be passionate about something (either way - for or against). The experience of doing it might well turn other kids off - but that's really the point of it. I bet nearly everyone who did that (we dissected earthworms and a cow's eye) remembers that hour of Biology class more vividly than almost any other day of their entire school lives. It's not about teaching anatomy any more than measuring the period of a pendulum in Physics classes is about learning that oh-so-not-vital length-versus-time equation that 99% of them will never use or remember again. It's an experience that no parent is ever likely to teach them...and that's precisely why you shouldn't be able to opt-out. That's also why we need to keep metalwork & woodwork class, art class and music classes, if kids never get to experience those things - how will they know whether they are passionate about them? SteveBaker (talk) 13:07, 5 November 2009 (UTC)
- It's hard to prove a counter-factual, though. How do we know that 1% wouldn't have found another path there? (Or that your 99% weren't so turned off by it that they decided science class wasn't for them?) Have we really proven in a rigorous way that dissection actually is a useful pedagogical tool, to the point where those who believe that it is just "unnecessary" killing of animals should be ignored? I don't know the answer, but that seems at issue here—you're assuming the memory of the spectacle itself translates into good pedagogy, but I'm not sure that necessarily follows. (Incidentally, my favorite take on classroom dissection is this one.) --Mr.98 (talk) 15:21, 5 November 2009 (UTC)
- Steve, you seem to have missed the central point of my post and commented solely on my anecdote. Killing things is usually seen as a "bad thing" unless the killing is justified. My point is that the line between "not justified" and "justified" is not the same for everyone at all times and people need to keep that in mind before they go off half-cocked about the choice regarding dissection being a "religion vs science" thing.
- Your comments about the impact high school dissection has on kids can also swing both ways - I would wager that for every kid that got turned on to biology by doing a dissection you would find at least an equal number of students who were traumatized at the very thought and therefore failed to go on to contribute to the studies of cladistics or ethology. While you and I obviously think back to those days as ones of discovery, there are a great number of people who think back to it and shudder at the very thought. Matt Deres (talk) 22:21, 5 November 2009 (UTC)
Bears can play hockey?
I watched an interesting video on the internet that shows a team of bears playing hockey. This is the internet so it can quite possibly be fake, but it at least looks real. What do you think? http://video.yahoo.com/network/100284668?v=6255496&l=4418225 if it says "video not available", bypass your cache. -- penubag (talk) 22:07, 4 November 2009 (UTC)
- Yes, according to ABC News, bears playing ice hockey is a standard stunt in the Russian circus.[9] Red Act (talk) 22:30, 4 November 2009 (UTC)
- That's crazy. I'd love to go see this in person! -- penubag (talk) 01:25, 5 November 2009 (UTC)
- Bypass your cache?218.25.32.210 (talk) 01:23, 5 November 2009 (UTC)
- WP:Bypass your cache -- penubag (talk) 01:24, 5 November 2009 (UTC)
- I remember seeing a hockey-playing bear at a Budapest circus, so it isn't just Russians (or it was a Russian traveling circus) Rmhermen (talk) 03:05, 5 November 2009 (UTC)
- Are they playing hockey or just holding sticks and hitting pucks? DRosenbach (Talk | Contribs) 03:44, 5 November 2009 (UTC)
- Are we talking ice hockey then? Nil Einne (talk) 09:26, 5 November 2009 (UTC)
- There are some bears in Boston and Providence that can play ice hockey. --Mark PEA (talk) 17:41, 5 November 2009 (UTC)
- They play football in Chicago.—Preceding unsigned comment added by Googlemeister (talk • contribs)
- In Chicago, you can watch wolves playing hockey. Edison (talk) 15:36, 6 November 2009 (UTC)
- They play football in Chicago.—Preceding unsigned comment added by Googlemeister (talk • contribs)
- There are some bears in Boston and Providence that can play ice hockey. --Mark PEA (talk) 17:41, 5 November 2009 (UTC)
- It's amazing how good they are. They're completing passes and everything, and actively trying to score goals. They seem reasonably comfortable ice-skating around in a bipedal fashion.
- White team needs a new goal-keeper, though. APL (talk) 17:48, 5 November 2009 (UTC)
- Could it be a combination of some real bears, people in costumes, computer graphics, and skillful editing? Today one sees lots of TV commercials with animals doing fake things. There are about 39 edits or cut between shots averaging every 3.5 seconds seconds in this 2 minute video, which would be an opportunity to insert closeups of fakery, or to intercut shots from different times to make it look like continuous play. It is also very fuzzy for a slick production with that much editing. This is no camcorder shot. Edison (talk) 15:43, 6 November 2009 (UTC)
- To me the fuzziness looks like it's come from many generations of video tape duplication. Presumably this was on TV at some point. Since there's a live audience, and this is supposedly a common stunt in Russia, I doubt that it's literally fake. I'm sure the editing improves it, but the crowd is loving it, so even without editing it must be pretty good. APL (talk) 20:43, 6 November 2009 (UTC)
- Could it be a combination of some real bears, people in costumes, computer graphics, and skillful editing? Today one sees lots of TV commercials with animals doing fake things. There are about 39 edits or cut between shots averaging every 3.5 seconds seconds in this 2 minute video, which would be an opportunity to insert closeups of fakery, or to intercut shots from different times to make it look like continuous play. It is also very fuzzy for a slick production with that much editing. This is no camcorder shot. Edison (talk) 15:43, 6 November 2009 (UTC)
- Are we talking ice hockey then? Nil Einne (talk) 09:26, 5 November 2009 (UTC)
- Are they playing hockey or just holding sticks and hitting pucks? DRosenbach (Talk | Contribs) 03:44, 5 November 2009 (UTC)
- I remember seeing a hockey-playing bear at a Budapest circus, so it isn't just Russians (or it was a Russian traveling circus) Rmhermen (talk) 03:05, 5 November 2009 (UTC)
- WP:Bypass your cache -- penubag (talk) 01:24, 5 November 2009 (UTC)
Harmful beryllium oxide in ceramic insolators
I was reading the article about microwave ovens here in Wikipedia, and there was written that some microwave oven magnetrons have ceramic insulators with a piece of harmful beryllium oxide (beryllia) added. How will a person know if the ceramic insulators with a piece of beryllia is a bit broken? Will the microwave oven keep on working if it is a little broken (or a little crushed)? If the ceramic insolator should be a liitle broken or crushed, can the dust from it get inside the cooking chamber (or outside of the microwave oven)?JTimbboy (talk) 22:46, 4 November 2009 (UTC)
- The OP probably refers to this text in the article Microwave oven: Some magnetrons have ceramic insulators with a piece of beryllium oxide (beryllia) added—these ceramics often appear somewhat pink or purple-colored. The beryllium in such oxides is a serious chemical hazard if crushed and ingested (eg, inhaling dust). In addition, beryllia is listed as a confirmed human carcinogen by the IARC; therefore, broken ceramic insulators or magnetrons should not be handled. This is obviously only a danger if the microwave oven becomes physically damaged (ie, cracked ceramics) or upon opening and handling the magnetron directly, and as such should not occur during normal usage. Cuddlyable3 (talk) 01:37, 5 November 2009 (UTC)
November 5
A sculptor who works in dark matter
How would such a sculptor go about it? What would the results look like? or, if invisible, is there any way one could perceive the results?
Thanks
Adambrowne666 (talk) 00:08, 5 November 2009 (UTC)
- Nobody even knows for sure what dark matter is, so there is no way anyone could make a sculpture out of it. --Tango (talk) 00:17, 5 November 2009 (UTC)
- True. Let's play with the idea though, based more on current theories than on what it is for sure. Adambrowne666 (talk) 00:20, 5 November 2009 (UTC)
- I think there are two main theories - either it is regular matter that is just too cold to radiate light (MACHOs), in which case it would be sculpted in the same way as any other regular matter, or it is "weakly interacting massive particles" (WIMPs) which means particles which only interact with other matter through gravity and the weak interaction (which is only really significant in radioactivity). Such matter couldn't be sculpted at all, since there would be no way to hold the dark matter together. --Tango (talk) 01:00, 5 November 2009 (UTC)
- Were those acronyms intentional? Seems too unlikely to be a mere conincidence... (MACHO and WIMP, fight!) --antilivedT | C | G 05:57, 5 November 2009 (UTC)
- I think there are two main theories - either it is regular matter that is just too cold to radiate light (MACHOs), in which case it would be sculpted in the same way as any other regular matter, or it is "weakly interacting massive particles" (WIMPs) which means particles which only interact with other matter through gravity and the weak interaction (which is only really significant in radioactivity). Such matter couldn't be sculpted at all, since there would be no way to hold the dark matter together. --Tango (talk) 01:00, 5 November 2009 (UTC)
- True. Let's play with the idea though, based more on current theories than on what it is for sure. Adambrowne666 (talk) 00:20, 5 November 2009 (UTC)
- Most theories of dark matter would have it be more like a gas (weakly interacting) than a solid, in which case there would be nothing to sculpt. Dragons flight (talk) 00:22, 5 November 2009 (UTC)
- And with so little interaction with normal matter, dark matter could not be confined in a gas tank, or cut with with a blade. The only way to control it is with gravity. In any case most of the matter on earth does not give off light, and could be considered dark matter. Graeme Bartlett (talk) 00:46, 5 November 2009 (UTC)
- Ok, but ferrofluid isliquid, and can be sculpted, in a manner of speaking. Say we use gravity to shape dark matter. What then? Is there any way of showing off our work? Adambrowne666 (talk) 01:01, 5 November 2009 (UTC)
- If you want to use gravity to hold it together you would need to work and store it in a zero-gee environment. If the dark matter is just regular matter then it would reflect light so you could show it off in the same way as any other sculpture. If it is just weakly interacting then I don't think there is any way it could be directly seen. --Tango (talk) 01:05, 5 November 2009 (UTC)
- Sounds a bit like it would have to be Conceptual art. AlmostReadytoFly (talk) 09:17, 5 November 2009 (UTC)
- If you want to use gravity to hold it together you would need to work and store it in a zero-gee environment. If the dark matter is just regular matter then it would reflect light so you could show it off in the same way as any other sculpture. If it is just weakly interacting then I don't think there is any way it could be directly seen. --Tango (talk) 01:05, 5 November 2009 (UTC)
- Ok, but ferrofluid isliquid, and can be sculpted, in a manner of speaking. Say we use gravity to shape dark matter. What then? Is there any way of showing off our work? Adambrowne666 (talk) 01:01, 5 November 2009 (UTC)
- Most art galleries today don't show off dark-matter sculptures. Also, many artists fail to be inspired by it as a working material. Bus stop (talk) 15:56, 5 November 2009 (UTC)
- Yes, probably right, Tango; there's no way it could be directly seen - aside from the twisted gravity field, is there any way its presence and form could be detected by a person in the same room? what equipment would such a person need to perceive the art? Adambrowne666 (talk) 09:46, 5 November 2009 (UTC)
- If it were heavy enough they could detect the gravity directly, especially in they were otherwise in zero-gee. If it were lighter then watching the paths of slow moving ball bearings as you throw them around the room might allow you to detect it, or some kind of smoke generator. --Tango (talk) 15:30, 5 November 2009 (UTC)
- Yes, probably right, Tango; there's no way it could be directly seen - aside from the twisted gravity field, is there any way its presence and form could be detected by a person in the same room? what equipment would such a person need to perceive the art? Adambrowne666 (talk) 09:46, 5 November 2009 (UTC)
- Question - assuming the sculptor can assemble this huge amount of dark matter (mass of a small asteroid if you want it to deflect the path of a ball bearing), how do they keep it in one place ? What stops it dropping straight through the floor ? Gandalf61 (talk) 15:40, 5 November 2009 (UTC)
- That's why it needs to be stored in zero-gee. Something that heavy would generally accelerate very slowly, so it shouldn't be too difficult to keep it central in the space-borne art gallery by moving the gallery to compensate for its movements. --Tango (talk) 19:52, 5 November 2009 (UTC)
- Question - assuming the sculptor can assemble this huge amount of dark matter (mass of a small asteroid if you want it to deflect the path of a ball bearing), how do they keep it in one place ? What stops it dropping straight through the floor ? Gandalf61 (talk) 15:40, 5 November 2009 (UTC)
Thanks, all - it's for a story I'm working on, and I think with a bit, or a lot, of fudging, I can work with this stuff to get something fun. Adambrowne666 (talk) 22:46, 6 November 2009 (UTC)
- Speculation: Perhaps you could assemble a ball of dark matter 1 decimeter across yet weighing millions of tonnes. This should have a measurable gravitational field. Or maybe it could condense into a streamer of dark matter held together by gravity, yet kept elongated by mixtures of velocities along the stream. Maybe you can sense the presence of darkmatter by its effect on dust or smoke in the air. A fast moving stream may have a gravitomagnetic effect and pull nearby matter in the same direction. Graeme Bartlett (talk) 06:27, 7 November 2009 (UTC)
What cognitive changes take place at birth?
There's a lot of controversy about when exactly killing a fetus/baby becomes murder. One common belief is that it's at (or possibly a little after) the moment of birth. This would only make sense if there are significant cognitive changes caused by being born. I wouldn't find it at all surprising, but it seems strange that I've never heard anything about it one way or another. Is it just impossible to test? If not, what changes take place? Is it generally considered by people in that field to be something necessary for sentience? — DanielLC 00:09, 5 November 2009 (UTC)
- I don't think there are any significant cognitive changes that abruptly occur at birth. However, at birth the baby goes from receiving nutrition and oxygen through the umbilical cord to breathing independently and requiring oral food. I know some people place special significance on that first breath, and on the independence from the systems of the mother. I don't think many people worry about sentience per se. Dragons flight (talk) 00:18, 5 November 2009 (UTC)
- If it were so, babies born by caesarean section would not be sentient. There is no sharp dividing line: babies born a little before 22 weeks have lived to be sentient, even though there are several important developments in the body and brain between even 34 weeks and the 'mature' 37 weeks. Really, how sentient do you consider a 4 week old baby? Does it have enough experience to understand the world well enough to be considered sentient? Time was, doctors regularly performed surgery on babies and little children without anaesthetic on the basis that they weren't mature enough to really feel pain: they were just reacting instinctively. We now know this is bunk (I know a few older ex-nurses who still go pale at the memory, horrified at what they had been doing once the evidence came in). I don't think there is consensus on a stage of development at which sufficient sentience has developed that the being can feel pain, and doctors carrying out pre-birth procedures have to balance many factors and possible outcomes when deciding what to do. Of course, they may be carrying out these procedures on babies who in other circumstances would be classified only as foetuses.
- But given we do not generally consider it murder to kill non-human animals, despite the increasing evidence (staring any observant human in the face) that many species are self-aware and really do suffer when in pain (possible definitions of sentience), I don't think it would really sway anyone. After all, we generally consider it far worse to kill a month-old child than a 5-year-old chimp, even if the chimp can demonstrate its sentience much more fully than the child. 86.142.224.71 (talk) 00:36, 5 November 2009 (UTC)
- And there is also the class of the severely retarded or mentally disabled who never pass into a "too little sentience, OK to kill" category (other than brain death). --Mr.98 (talk) 01:07, 5 November 2009 (UTC)
- Here you're confounding sentience with cognitive power. They're quite different conceptually. Sentience is the capacity to experience qualia. I see no reason to think that the severely mentally disabled are less sentient than the rest of us. Of course, by the very nature of qualia, we can never really know, one way or another — see solipsism and animism for two opposite conclusions on the question, neither of which is really susceptible to scientific inquiry. --Trovatore (talk) 04:41, 5 November 2009 (UTC)
- And there is also the class of the severely retarded or mentally disabled who never pass into a "too little sentience, OK to kill" category (other than brain death). --Mr.98 (talk) 01:07, 5 November 2009 (UTC)
- My belief is that people define words like "life" and "murder" in such a way as to support the rules they want people to follow -- that is, people start with the conclusions, and then work out the premises that are needed to derive those conclusions. Looie496 (talk) 02:30, 5 November 2009 (UTC)
- I'd argue that the initial assertion is not accurate. I don't know of anyone or any culture who believes killing a baby in utero a week before it is due would not be murder. Even the most ardent pro choice supporters I know would not argue it is fine to kill a baby just before it is born. A far more commenly held belief in the past was that the quickening was an indicaton of when a foetus became a real person with a soul, there fore capable of being murdered. Vespine (talk) 04:18, 5 November 2009 (UTC)
- The following is not given as legal advice. But contrary to the assertion by Vespine, many legal jurisdictions in the U.S. formerly required that a baby be born alive for its subsequent killing to be murder. Killing of a fetus=murder is a modern legal development in the U.S. See a legal textbook on "born alive" statutes:[10]. Under common law, killing of a fetus was not homicide. Edison (talk) 19:50, 5 November 2009 (UTC)
- According to Late-term abortion#Legal restrictions on later abortion, in 54 of the 152 most populous nations, abortion is legal at any stage. — DanielLC 05:48, 5 November 2009 (UTC)
- That section is a bit nebulous. If you read the reference associated with that section's content, it's not entirely clear that that math is exact. The review groups countries into how they are restricted on availability, such as to save the woman's life or for socioeconomic reasons; a gestational limit isn't one of those categories. A gestational limit is only noted for countries with no other restrictions and of those, only four have laws that don't specify a limit - Canada, China, North Korea, and Vietnam. So, other countries may or may not have gestational limits regulating abortions in addition to their other restrictions, but there's no way to tell based on that reference. Mind you, all of that is from 12 years ago, so many things may have changed. ~ Amory (u • t • c) 15:16, 5 November 2009 (UTC)
- I don't mean the process of birth itself. There is are a lot more sensations when you're born. Things actually happen. Before you're born, there's no point in having any significant brain activity. By the way, I was reading the abortion page and it said that there's some controversy that fetuses might feel pain. This seems rather silly, as they must be sentient to feel pain, and if they are, it's bad to kill them because it's murder. Is the idea that they're like animals for a period, and they have sentience, but no right to life? — DanielLC 05:48, 5 November 2009 (UTC)
- Yes. And babies do have significant brain activity before birth and it may be meaningful and they feel pain by most definitions. It's all about definitions really so people who work in words feel okay about it as we have no objective way of measuring sentience or pain and there is no objective reason for them to mean anything anyway, they are not like the amount of energy one can get from a lump of coal or anything like that. Dmcq (talk) 12:23, 5 November 2009 (UTC)
Maximum rate of a energy delivered for a given voltage
I am trying to understand if, given a voltage and a duration, whether it is possible to calculate the maximum amount of electrical energy that can be delivered. To put this in context I am trying to judge the maximum amount of energy it might take to recharge this car given just the voltage and the duration of energy delivery (100 volts - 180 minutes, 200 volts - 100 minutes). I understand that the voltage does not have an exact correlation with how much energy is drawn (my kettle draws 2kwh, and my phone charger much less, even though they are both plugged into a 240v socket), but I want to understand whether there is a maximum rate of energy transfer for a given voltage (or do other things, current?, come into play). Sorry, electricity has always been a big blind spot for me. Any help appreciated. BurningFridge (talk) 00:30, 5 November 2009 (UTC)
See the article on Electric power. In summary (and ignoring power factor) the energy transfer is a function of voltage, current and time. Power (watts) = voltage (volts) x current (amps); energy (joules) = power (watts) x time (seconds). Thus for any given voltage, the maximum energy transfer is a function of the current. In Australia, normal household power outlets are rated at a maximum 10A, although you can get 15A sockets. Mitch Ames (talk) 01:01, 5 November 2009 (UTC)
- The likely reason for the disparity (twice the voltage but not half the time) is likely to be to do with the need to limit battery overheating and perhaps also due to inefficiencies through the charging circuit which may worsen as the voltage gets bigger. The physics of recharging batteries is rather complicated. SteveBaker (talk) 01:14, 5 November 2009 (UTC)
- Some people find the Hydraulic analogy helps understanding of electricity. The difference between the OP's kettle and phone charger is that the kettle has much lower resistance R (ohms) than the charger. The power taken from the 240V supply is inversely proportional to R so the kettle takes the greater power. (We can calculate the kettle resistance R = 240 x 240 / 2000 = 28.8 ohm.) In principle, from a given voltage one can draw any power by connecting a load of appropriate R. In practice the power delivery is stopped if current I = 240 / R (amps) exceeds the current rating in amps of the fuse. In order to calculate the energy (joules) = power (watts) x time (seconds) for charging the car one needs to know the current drawn from the supply during the charge cycle. Cuddlyable3 (talk) 01:28, 5 November 2009 (UTC)
- Indeed, that's true. But it doesn't help much because those car chargers that attempt to charge as quickly as possible don't draw (or supply) continuous amounts of constant current - they have to monitor battery temperature and voltage and vary the amount of current as the batteries are charged in order to prolong their life while minimizing the recharge time. Therefore the actual recharge time will depend on the ambient temperature and the amount of ventilation keeping the batteries cool. It's not easy to calculate without understanding the details of what the charger is doing, how the battery temperature changes, how heat is lost from the battery compartment, etc. SteveBaker (talk) 12:50, 5 November 2009 (UTC)
- That's also true. To find the energy consumed one must measure how the current varies as a function of time during the charge cycle and integrate (i.e. find the area under the i = f(t) curve) to get the overall current x time product. To find the energy actually delivered to the battery one has to integrate over time the product of charge current and voltage, both varying. Energy delivered to the battery is less than energy consumed because inefficiency of the charger wastes the difference as heat. During use the battery will again deliver less energy than that with which it was charged. Cuddlyable3 (talk) 19:42, 5 November 2009 (UTC)
- From purely theoretical considerations of the physics of electricity, any given voltage could deliver any given amount of energy in a given time, if the circuit impedance were made low enough. The impedance of the load and the impedance of the source are what limit the current. Additionally the fuse or circuit breaker in the supply, along with the electronics of the battery charging circuit are the practically limiting variables. Edison (talk) 19:45, 5 November 2009 (UTC)
- That's also true. To find the energy consumed one must measure how the current varies as a function of time during the charge cycle and integrate (i.e. find the area under the i = f(t) curve) to get the overall current x time product. To find the energy actually delivered to the battery one has to integrate over time the product of charge current and voltage, both varying. Energy delivered to the battery is less than energy consumed because inefficiency of the charger wastes the difference as heat. During use the battery will again deliver less energy than that with which it was charged. Cuddlyable3 (talk) 19:42, 5 November 2009 (UTC)
- Indeed, that's true. But it doesn't help much because those car chargers that attempt to charge as quickly as possible don't draw (or supply) continuous amounts of constant current - they have to monitor battery temperature and voltage and vary the amount of current as the batteries are charged in order to prolong their life while minimizing the recharge time. Therefore the actual recharge time will depend on the ambient temperature and the amount of ventilation keeping the batteries cool. It's not easy to calculate without understanding the details of what the charger is doing, how the battery temperature changes, how heat is lost from the battery compartment, etc. SteveBaker (talk) 12:50, 5 November 2009 (UTC)
- Some people find the Hydraulic analogy helps understanding of electricity. The difference between the OP's kettle and phone charger is that the kettle has much lower resistance R (ohms) than the charger. The power taken from the 240V supply is inversely proportional to R so the kettle takes the greater power. (We can calculate the kettle resistance R = 240 x 240 / 2000 = 28.8 ohm.) In principle, from a given voltage one can draw any power by connecting a load of appropriate R. In practice the power delivery is stopped if current I = 240 / R (amps) exceeds the current rating in amps of the fuse. In order to calculate the energy (joules) = power (watts) x time (seconds) for charging the car one needs to know the current drawn from the supply during the charge cycle. Cuddlyable3 (talk) 01:28, 5 November 2009 (UTC)
Flame tests
Will metal ions with different charges (i.e. Fe3+ and Fe2+) produce different colors in flame tests? 76.204.127.175 (talk) 03:53, 5 November 2009 (UTC)
- That's a good question. If you haven't already, see emission spectrum. In this situation, it might be helpful to imagine a Bohr model. Under controlled circumstances, we should say that the tempurature is the same for both tests, because varying temperatures can effect the color. Fe+2 is the ion of iron, with 3 energy levels. When excited, we could say that the electrons jump up 3 energy levels, then fall to their original orbital energy level, releasing a photon of light. This is complexely measured in an emission spectrum.

- Let's say that for some reason, an electron was taken from the atom of iron, making it Fe+3. If this was able to be maintained, (it would be somewhat difficult to keep the unstable Fe+3 from reacting, unless in a container of inert gas) and it was excited, the electrons would only manage to jump up 2 energy levels. To the naked eye, one might not be able to distinguish different colors, but the emission spectrum would be the tell-all. It would be slightly different than a Fe+2 ion. If you have any questions, ask here or on my talk page :). Letter 7 it's the best letter :) 13:51, 5 November 2009 (UTC)
- Also when we are calm we could say that the electrons, when excited, jump up 3 energy levels. Cuddlyable3 (talk) 19:23, 5 November 2009 (UTC)
What is the capacitance of a black hole?
The article "Detecting Energy Emissions from a Rotating Black Hole" at [11] (free subscription required to view) makes the statement in passing that the capacitance of a black hole C = 1/M (approximately). I guess these are in natural units. Does anyone happen to know where this relationship is derived or explained, and what the units are? The Wikipedia article on natural units [12] doesn't define capacitance or voltage.
Trevor Turton —Preceding unsigned comment added by Tijaska (talk • contribs) 04:17, 5 November 2009 (UTC)
- This abstract says "The notion of electric capacitance for a black hole is introduced", so might be worth a look. --Sean 17:47, 5 November 2009 (UTC)
- Naively, I'd expect it to be similar to the self-capacitance of an isolated sphere, , where R is the Schwarzchild radius, but your reference suggests that's not even the right proportionality. So obviously more complicated effects matter. Dragons flight (talk) 18:32, 5 November 2009 (UTC)
- Natural units of charge are chosen so that G = 1/4πε0. With all factors added back in, C = 1/M should become C = 4πε0ħ / cM, but you probably shouldn't take my word on that. Membrane paradigm has a tiny bit on this subject and lists some references. -- BenRG (talk) 18:43, 5 November 2009 (UTC)
cosmatics
How cosmatics are manufactured, tell me complete procedure —Preceding unsigned comment added by 119.152.59.27 (talk) 06:36, 5 November 2009 (UTC)
I have slightly reformatted your question to make it easier to read. Richard Avery (talk) 08:30, 5 November 2009 (UTC)
- What kind of cosmetics? There are lots of different types. AlmostReadytoFly (talk) 09:12, 5 November 2009 (UTC)
- It's not very different from any other chemical product manufacture. Generally, a pigment (coloring chemical) is added to a binder (like a wax or a synthetic oil - or an alcohol, in some cases); fragrance may also be added. Finally, the stock mixture will be cut and packaged by special-purpose machinery. The actual procedure will depend on the type in question. Hopefully a quality-control process is in place to help ensure the safety of the finished product. Nimur (talk) 17:55, 5 November 2009 (UTC)
Insolation
How to find solar insolation patterns at various points in the jupiter's atmosphere. A comparison with earth is needed —Preceding unsigned comment added by 220.225.125.246 (talk) 09:16, 5 November 2009 (UTC)
- Figuring out the insolation at the top of the atmosphere is easy. It looks like all the trigonometry is worked out in the insolation article, whereas the parameters of Jupiter orbit are given, not surprisingly, in the Jupiter (planet) article. Now, the insolation within the atmosphere is very much more tricky. Strictly speaking, you need to model the absorption and the scattering of light at every position at every wavelength into every direction. This requires a full 3D radiation transfer model. As Gandalf said, "this foe is beyond any of you..." --Dr Dima (talk) 10:32, 5 November 2009 (UTC)
Properties of alcohols
what are he properties for the following alcohols and their chemical formulae:
- DL-2-pentanol
- pentan-1-0l
- octanol-1-0l
- ethanol
- butan-1-ol
- n-porpyl
send to email address removed —Preceding unsigned comment added by 120.18.247.221 (talk) 11:40, 5 November 2009 (UTC)
- Wikipedia gets copied mercilessly across the internet. It's a bad idea to put your email address on this website, as it will likely attract much more spam than you want. Falconusp t c 12:39, 5 November 2009 (UTC)
- We do not answer questions by email - you have to return here to find responses - and we remove people's email addresses if they provide them. We have articles on pentanol, octanol, ethanol, butanol and propanol which (I believe) will answer all of your questions. SteveBaker (talk) 12:42, 5 November 2009 (UTC)
- (edit conflict)
- See Amyl alcohol
- See Amyl alcohol
- See octanol
- See ethanol
- See butanol
- propyl?
- These pages generally give the formulae and properties. AlmostReadytoFly (talk) 12:49, 5 November 2009 (UTC)
Uncompressed image file formats other than .bmp files
What other file formats are there, apart from BMP files, that are uncompressed and can be processed as a raw bit stream? I require to know the exact file structure of these formats.Csanghamitra (talk) 15:34, 5 November 2009 (UTC)
- The results for searching this includes:
- This question belongs on the Computing desk, I think. Imagine Reason (talk) 16:22, 5 November 2009 (UTC)
- TIFF files are commonly used by image processing people. They can store uncompressed data. PNG can store with lossless compression (but compressed), depending on whether your software tool supports this feature. Our Comparison of graphics file formats article allows you to sort by compression technique. If you're looking specifically for no compression, your options are narrowed down pretty significantly; lossless compression preserves information but is a bit more work for you as a programmer. "Anything" can be handled as a bitstream, but it sounds like you want to be able to seek to a specific pixel location without decoding any other values - that is a bitmap by definition; and you probably want a .BMP or TIFF container format. Nimur (talk) 18:02, 5 November 2009 (UTC)
- There aren't many formats that guarantee uncompressed data. PNG and TIFF are probably the best choices for LOSSLESS compression (as opposed to GIF and JPEG which have LOSSY compression). But I'm struggling to think of any format that has no compression whatever. The old "SGI" image format has only run-length encoding - it's relatively easy to unpack yourself. If you use the GIMP package for saving files, it has a "Save" format called "Raw image data" that is literally just a bunch of bytes with the pixels in it...that's guaranteed to be uncompressed and has no "structure" whatever. These days, most people just grab the freeware libPNG or libTIFF libraries and let them do the unpacking. Both can deliver a simple array of bytes to your application code. SteveBaker (talk) 18:44, 5 November 2009 (UTC)
- No. Strictly speaking the GIF format gives compression with NO LOSS and certainly nothing like the distortion artifacts that strong JPEG compression produces. It is true that the number of different colours in an image may need to be reduced to keep within the maximum 256 colours that GIF can handle, but beyond that the GIF format has LOSSLESS compression. Cuddlyable3 (talk) 19:02, 5 November 2009 (UTC)
- Well, yes - in the special case where your image happens to have that few colors and the conversion tool makes up a palette on-the-fly rather than using a standardized one...you're right - but those are very special circumstances. In general, it's lossy. SteveBaker (talk) 22:02, 5 November 2009 (UTC)
- This is why lossy compression is often defined in terms of generational loss—otherwise you risk having to classify every format as lossy because it demands a grid of pixels and at most 256 levels per channel and so on. GIF is only defined for input images of 256 colors or less, and compresses those without loss, so it's normally classified as lossless. At some level it's all relative; you could define the input of MP3 as just those waveforms that can be produced by a reference decoder, and a good enough lossy encoder can avoid all generational loss (most encoders in the wild are not that good). But GIF is normally considered lossless. -- BenRG (talk) 22:37, 5 November 2009 (UTC)
- Well, yes - in the special case where your image happens to have that few colors and the conversion tool makes up a palette on-the-fly rather than using a standardized one...you're right - but those are very special circumstances. In general, it's lossy. SteveBaker (talk) 22:02, 5 November 2009 (UTC)
- No. Strictly speaking the GIF format gives compression with NO LOSS and certainly nothing like the distortion artifacts that strong JPEG compression produces. It is true that the number of different colours in an image may need to be reduced to keep within the maximum 256 colours that GIF can handle, but beyond that the GIF format has LOSSLESS compression. Cuddlyable3 (talk) 19:02, 5 November 2009 (UTC)
- Vector graphic formats are compact and unsuitable for lossy compression.
The OP does not exclude mentioning the uncompressed audio formats WAV and PCM.Cuddlyable3 (talk) 19:15, 5 November 2009 (UTC)
- Vector graphic formats are compact and unsuitable for lossy compression.
- The OP did specify image file formats. (I'm unclear why bringing in vectors is relevant... it seems rather clear they mean bitmaps). --98.217.71.237 (talk) 20:37, 5 November 2009 (UTC)
- Thank you, you are right and I strike the mention of audio. Vector graphic image formats are examples of uncompressed image file formats, so they are relevant to the OP who excluded one but did not insist on only raster formats. Cuddlyable3 (talk) 00:41, 6 November 2009 (UTC)
- A very nice format -- because of its openness, simplicity, and the number of tools available for manipulating it -- is NetPBM. —Steve Summit (talk) 23:27, 5 November 2009 (UTC)
- TGA images can be stored uncompressed (and BMP supports run-length compression, though almost nobody uses it). Various digital camera raw formats are also uncompressed, but it takes a good deal of post-processing to turn them into usable images. --Carnildo (talk) 23:47, 5 November 2009 (UTC)
Sticking finger
Why do we feel sticky when we press a finger on frozen ice in the freezer of a refrigerator? —Preceding unsigned comment added by Cssivakumar (talk • contribs) 18:51, 5 November 2009 (UTC)
- Because your finger has slightly frozen to the ice. More fully, the heat from your finger slightly melted the ice. However, if you touch something sufficiently cold, the surface of your finger loses heat faster than the whatever-it-is gains heat, and the just-melted water quickly re-freezes. — Lomn 19:13, 5 November 2009 (UTC)
- The effect is much greater if your finger is already wet - that leads to cold burns. --Tango (talk) 19:48, 5 November 2009 (UTC)
Solitude drove the old hermit crab insane.
Are there any hermit crabs that actively kill shell-living critters for their houses? Vitriol (talk) 18:52, 5 November 2009 (UTC)
- They don't unless they have to. If they cannot find a discarded shell they will attack a shelled-creature and nick their house. It's usually only a problem in home aquariums, as the natural environment is littered with suitable "crabitation". Fribbler (talk) 19:00, 5 November 2009 (UTC)
'Diesel' engine
Trying to make sense of second paragraph in [A http://railroad.100megsfree5.com/L8/GazTurbo-GT101.html] , the engine may be called 'СПГГ' (russian/cyrllic type) [B http://images.google.co.uk/images?hl=en&q=%D0%A1%D0%9F%D0%93%D0%93&um=1&ie=UTF-8&sa=N&tab=wi] - is the loco engine same type as here [C http://dic.academic.ru/dic.nsf/ruwiki/1140871].?
Also does this type have an article / english name? Following on I'd like to ask if anyone knows how the fuel/air (?) is injected into 'small cylinder' following release of pressurised combusted gas into turbine as shown in third link (C above) - there must be some sort of 'tappet valve' activated on return stroke to allow recharge of fuel air mixture? anyone know? Thanks..83.100.251.196 (talk) 18:53, 5 November 2009 (UTC)
- We have Gas turbine locomotive and Gas turbine-electric locomotive. Commons has a picture of the GT101, but only the Russian Wikipedia links to it. We have an article GT 101, which appears to be a similar German -- not Russian -- thing, but perhaps they're related. --Sean 20:33, 5 November 2009 (UTC)
- Thanks (I think GT is gas turbine in many languages and GT 101 is a coincidence) - it seems it's more common that I though - part of it is called a Free-piston engine - it was the valve or equivalent arrangements in this engine that I was wondering about.83.100.251.196 (talk) 22:45, 5 November 2009 (UTC)
- I think I've found the answer here [15] fig 12.5 it looks like they are simple one way valves.
- It didn't think to look at gas turbine locomotive - I thought it was to obscure to be mentioned..but there were similar things in france and sweden...
- If there is a name (person?) for the "free piston / gas turbine combination" it would be interesting, or is the free piston engine always assumed coupled to a gas turbine??83.100.251.196 (talk) 22:50, 5 November 2009 (UTC)
Drosophila melanogaster and human genome homology
Is there a published estimate of how many similar genes we and the fruit fly have? The Drosophila melanogaster article has some numbers, but I contested them on the talk page years ago, sadly without good sources. Now a prominent Finnish newspaper stated on their science pages that the genetic similarity is 66%, but that I just can't make myself believe (and therefore I hesitate to use that as a reference in the Finnish featured DM article) Any ideas about reliable homology percentage estimates? --Albval (talk) 20:25, 5 November 2009 (UTC)
- Actually, I'm surprised it's so little. Remember that most of that genome is comprised of things to make cells run - all sorts of complicated biochemistry that's very similar - if not identical - between a fruit fly and a human. SteveBaker (talk) 21:58, 5 November 2009 (UTC)
- I did a little research and found the BBC saying it was 60% (but no reliable sources, really). I'm a little surprised it is so low too - I've heard similar figures for bananas and surely we have a lot more in common with fruit flies than bananas. There are different ways to measure genetic similarity, though. For example, do you include differences in non-coding DNA or not? --Tango (talk) 22:26, 5 November 2009 (UTC)
- Well, they are fruit flies. ~ Amory (u • t • c) 22:39, 5 November 2009 (UTC)
- It wouldn't be possible to compare non-coding regions between fruit flies and humans -- there's no way to figure out what a given part should be compared to. Looie496 (talk) 23:58, 5 November 2009 (UTC)
- It's possible to compare the non-coding regions that are linked to a particular gene, such as introns. It's also possible to compare non-coding regions of more recently diverged organisms, such as humans to mice, as the sequence of chromosomal rearrangements can be deduced, and the non-coding regions aligned. However, you are correct, that there would be no way to know how to align the non-coding regions of humans and fruit flies. Anyway, I've been trying to find something, anything, to answer the OP's question. As with others, I have found some news reports that give a number, but I haven't found any reliable sources on the matter outside of research reports related to only a specific gene, and not the whole genome. Someguy1221 (talk) 01:10, 6 November 2009 (UTC)
- It wouldn't be possible to compare non-coding regions between fruit flies and humans -- there's no way to figure out what a given part should be compared to. Looie496 (talk) 23:58, 5 November 2009 (UTC)
- Well, they are fruit flies. ~ Amory (u • t • c) 22:39, 5 November 2009 (UTC)
- I did a little research and found the BBC saying it was 60% (but no reliable sources, really). I'm a little surprised it is so low too - I've heard similar figures for bananas and surely we have a lot more in common with fruit flies than bananas. There are different ways to measure genetic similarity, though. For example, do you include differences in non-coding DNA or not? --Tango (talk) 22:26, 5 November 2009 (UTC)
77% of human disease genes have versions or "cognates" in Drosophila.[16] Fences&Windows 04:12, 6 November 2009 (UTC)
- I haven't checked in a few years. 60-77% sounds correct as a rough estimate. I'll go see if I can scare up some better numbers. -- Flyguy649 talk 05:50, 6 November 2009 (UTC)
- The 2003 Drosophila fly community White Paper states that greater than 60% of Drosophila genes have human counterparts with the 70% figure describing similarities to disease-associated genes [17]. I'll see if there's anything more recent, but I'd be surprised if it's in a paper. This degree of conservation between flies and humans was really surprising when it was first discovered in the early part of the millennium. Now it's accepted as fact. At the time of the big genome sequencing projects, the estimate for the number of human genes ranged from 50,000-100,000. Researchers were shocked to find that there are only 23,000 or so genes in humans (flies have ~14,000) .-- Flyguy649 talk 06:18, 6 November 2009 (UTC)
- Feels somewhat strange to me that there aren't any more detailed comparative analyses made, especially when you see different kinds of human-mouse and human-fly similarity percentages in the popular media quite often. Or is it computationally just too hard to figure out, which DNA stretches actually are homologous? Or is it just not scientifically interesting enough? Anyway, your estimate is good enough for me, the exact percentage the newspaper article gave just made me wonder if there really was a study wrom which the newspaper article had taken its figures. Now it seems that the journalist just took the numbers out of thin air... --Albval (talk) 08:43, 6 November 2009 (UTC)
- The 2003 Drosophila fly community White Paper states that greater than 60% of Drosophila genes have human counterparts with the 70% figure describing similarities to disease-associated genes [17]. I'll see if there's anything more recent, but I'd be surprised if it's in a paper. This degree of conservation between flies and humans was really surprising when it was first discovered in the early part of the millennium. Now it's accepted as fact. At the time of the big genome sequencing projects, the estimate for the number of human genes ranged from 50,000-100,000. Researchers were shocked to find that there are only 23,000 or so genes in humans (flies have ~14,000) .-- Flyguy649 talk 06:18, 6 November 2009 (UTC)
- Coincidentally, I was reading The Greatest Show on Earth: The Evidence for Evolution last night and Dawkins makes a good point about these percentage-identical measurements for DNA. As he explains, if you're comparing the individual C, G, A and T base-pairs - then you get a different answer depending on how you handle missing, inserted or repeated sequence comparisons. If you are talking about codons, you get another different answer because there are cases where two or more different codons produce identical proteins - do you count those as "different" or not? If you are talking about genes - then you get another different answer because two genes only have to differ by one letter to be "different" and that means that there is a much greater probability of two genes being different than two base-pairs, so the percentage-different numbers are much higher. If you compare entire chromosomes then the probability of any two creatures - even closely related members of the same species - being anything other than 100% different is almost zero. Worse still, some animals have much the same genes - but arranged on totally different chromosomes - are those very different or very similar? So unless you are VERY careful about the level of comparison and the method of accounting for differences - then you can't give a definitive answer. That means that the 'popular-science' answers like "Chimpanzees and Humans have 98% identical DNA" doesn't mean very much.
- Consider, for example, the following two DNA chunks (evidently taken from feline DNA!):
CATCATCATCATCATCATCA...
CATGATCATCATCATCATCA...
^
- They clearly differ in only one letter - and also only in one codon (a codon is a group of 3 letters). But what about:
CATCATCATCATCATCATCA...
CATATCATCATCATCATGCA...
^ ^
- These differ by the deletion of one base-pair (a 'C') near the beginning and the insertion of another letter (a 'G') near the end. Should we say that they are nearly identical because they differ by only two base-pairs? Well, not really - the codons in the middle are completely different and would produce radically different proteins. So do these two animals have 90% identical DNA (two changes in 20 letters) or are they only 30% identical (14 differences in 20 letters) or are they 0% identical (because these two chunks would code for completely different proteins)?
- I see the difficulty in base-pair/codon -level comparisions, but homologous genes shouldn't (in my opinion) be too hard to find. There will be differences even in the amino acid level of course, but the "gene" is the same. So basically if you could find all the genes in the fruit fly and in human that have shared ancestry and then divide that by total, you'd get the "popular science" answer. I'm just wondering if it is too hard to do or too silly to do, because it seems that nobody really has done it? Or am I just missing something? Albval (talk) 18:58, 6 November 2009 (UTC)
Hydrogen orbital average radius in terms of energy level.
What is the mathematical relationship between the average radius of an electron's orbit around a proton in terms of the electron's energy level? -Craig Pemberton (talk) 22:48, 5 November 2009 (UTC)
- Average radius is [ 3n^2 - l(l+1) ] / 2 in atomic units. Here n is principal quantum number, l is orbital quantum number. Is that what you are asking? --Dr Dima (talk) 22:55, 5 November 2009 (UTC)
- (edit conflict) It starts with Electric_potential#Electric_potentials_due_to_point_charges (this gives the relationship between potential energy and distance) - but to be accurate you need an average weighted properly by the probability distribution of the electron's likely hood at a given distance. (ie a mean average is not good) - that is the classical method to do it. You could just use a mean average anyway..83.100.251.196 (talk) 22:58, 5 November 2009 (UTC)
- For the Bohr-model hydrogen atom, rn = n2 * (5.30 x 10-11 m) where:
- rn = the radius of energy level with principle quantum number n
- There is also likely a more general equation for calculating the radius of any one-electron atom (such as He+1 and Li+2), but that is the one I found for the Bohr-model hydrogen atom. Note that the Bohr model is a pretty limited model. It works VERY well for one electron systems because it is basically a two-body problem. For any system with 2 or more electrons, you have a n-body problem, which is a chaotic system and where you cannot mathematically predict things like energy level radius and even the energies of those levels directly. --Jayron32 04:46, 6 November 2009 (UTC)
- For the Bohr-model hydrogen atom, rn = n2 * (5.30 x 10-11 m) where:
November 6
Al
What is Al on the periodic table -nick —Preceding unsigned comment added by 76.199.148.217 (talk) 01:00, 6 November 2009 (UTC)
- Did you look for Al at Periodic table ? Perhaps Friday is not a good day for you. Cuddlyable3 (talk) 01:07, 6 November 2009 (UTC)
- Based on the IP address, I think it's still Thursday for that user. -- Scray (talk) 04:51, 6 November 2009 (UTC)
- Some spell it "Aluminum." Edison (talk) 04:54, 6 November 2009 (UTC)
- Based on the IP address, I think it's still Thursday for that user. -- Scray (talk) 04:51, 6 November 2009 (UTC)
- Did you look for Al at Periodic table ? Perhaps Friday is not a good day for you. Cuddlyable3 (talk) 01:07, 6 November 2009 (UTC)
- Searching is quicker for something like this. One can put 'Al element' or 'chemical Al' or 'AL periodic table' in and have a look at the results. Or one can put in 'Periodic table' and use CTRL+F and type 'A' in the browser search box. Or one can put these search terms into google. All quite quick and well worthwhile learning. Dmcq (talk) 12:01, 6 November 2009 (UTC)
- In fact I just tried 'Al' on its own in google and Aluminium in Wikipedia was the first entry returned Dmcq (talk) 12:03, 6 November 2009 (UTC)
progenitor cells
I have read a bit about using skin cells instead of stem cells, and that there has been a lot of success with this approach, it also seems as though there are a few research groups doing groundbreaking work with skin cells. Anyways, I was wondering if skin cells have or if progenitor cells could be derived from skin cells, just as they can be derived from stem cells? —Preceding unsigned comment added by 71.156.167.117 (talk) 01:39, 6 November 2009 (UTC)
- The skin certainly does have stem and progenitor cells, but you are probably referring to induced pluripotent stem cells. --- Medical geneticist (talk) 02:11, 6 November 2009 (UTC)
- Actually, not many people know it -but today when one is going through an accident which result with a clear cut of his/her spinal cord -an accident that ones meant one result only : clear and constant disability (usually paraplegic)-today there is a new treatment available that is regulary used in few places around the globe. This treatment involve with the insertion of stem cells to location of the injury and it must be done within 48-72 hours from the injury itself. This treatment have about 100% success. The stem cells are achived from mature epidermic cells by removing methyl groups which keep the wanted master genes unactive and later shuttering others and so we can extract from mature cells culture a culture of stem cells that will go through cellular differentiation process to become new spinal cord tissues . --Gilisa (talk) 08:14, 6 November 2009 (UTC)
- Gilesa, you are no doubt describing the possible use of iPSCs or other such induced pluripotent cells to treat spinal injury, but to my knowledge this is still a theory and not a proven medical treatment. Can you provide a reference? Just because an experimental therapy is being tried somewhere does not mean it is a proven success. There has been a great deal of hype around stem cell therapeutics, with "miracle cures" being claimed by people doing rather unscientific experimental treatments. Most of the mainstream literature still uses animal models of spinal cord injury, we don't really know the long-term effects of injecting stem cells into the spinal cord, and to my knowledge there has been no convincing clinical trial in humans, otherwise this would be a mainstream therapy. Sorry, but I'm a natural skeptic and need to see the data before I'm willing to accept that what you say ("about 100% success") is correct. --- Medical geneticist (talk) 14:18, 6 November 2009 (UTC)
- I have to agree with Medical geneticist. I know rather well someone who has been a paraplegic since being injured in a motorcycle accident (truck turning didn't see him) for slightly over 3 years so have some personal experience here. Stem cells have shown some promise in improving regeneration for people with spinal cord injuries; and there are places where lower levels of regulation mean that they are 'regularly' (well I mean more commonly then the occasional trial that happens in most of the developed world) tried (for a variety of things). However I'm not aware of any evidence for 100% effectiveness in preventing paraplegic and find that extremely unlikely in theory and the lack of any mention of this in most sources also makes it very unlikely. As MG said, if it was 100% effective you can bet it would be a more mainstream therapy, or at the very least there would be a lot more hype and knowledge about this. (Why would someone hide such stellar results?) I personally suspect we will one day be able treat spinal cord injuries resonably effectively (whether primarily because of stem cells or something else) although suspect (unfortunately for my friend, although he also suspects this is the case) early results will be with those with recent injuries not who have had their injuries long term and we will probably never be able to treat long term injuries as effectively. Back to the main point, many of the current trials do of course concentrate on recent injuries although looking at the sources (particularly the last two) it doesn't appear 48-72 hours is the optimal time frame currently rather 1-2 weeks. [18] [19] [20] [21] [22] [23] [24] Nil Einne (talk) 08:02, 7 November 2009 (UTC)
- Well, it seems like your sources are more up to dated than me. And it also seems like a friend, who is into stem cells research, fooled me once again.:(--Gilisa (talk) 16:23, 7 November 2009 (UTC)
- I have to agree with Medical geneticist. I know rather well someone who has been a paraplegic since being injured in a motorcycle accident (truck turning didn't see him) for slightly over 3 years so have some personal experience here. Stem cells have shown some promise in improving regeneration for people with spinal cord injuries; and there are places where lower levels of regulation mean that they are 'regularly' (well I mean more commonly then the occasional trial that happens in most of the developed world) tried (for a variety of things). However I'm not aware of any evidence for 100% effectiveness in preventing paraplegic and find that extremely unlikely in theory and the lack of any mention of this in most sources also makes it very unlikely. As MG said, if it was 100% effective you can bet it would be a more mainstream therapy, or at the very least there would be a lot more hype and knowledge about this. (Why would someone hide such stellar results?) I personally suspect we will one day be able treat spinal cord injuries resonably effectively (whether primarily because of stem cells or something else) although suspect (unfortunately for my friend, although he also suspects this is the case) early results will be with those with recent injuries not who have had their injuries long term and we will probably never be able to treat long term injuries as effectively. Back to the main point, many of the current trials do of course concentrate on recent injuries although looking at the sources (particularly the last two) it doesn't appear 48-72 hours is the optimal time frame currently rather 1-2 weeks. [18] [19] [20] [21] [22] [23] [24] Nil Einne (talk) 08:02, 7 November 2009 (UTC)
- Gilesa, you are no doubt describing the possible use of iPSCs or other such induced pluripotent cells to treat spinal injury, but to my knowledge this is still a theory and not a proven medical treatment. Can you provide a reference? Just because an experimental therapy is being tried somewhere does not mean it is a proven success. There has been a great deal of hype around stem cell therapeutics, with "miracle cures" being claimed by people doing rather unscientific experimental treatments. Most of the mainstream literature still uses animal models of spinal cord injury, we don't really know the long-term effects of injecting stem cells into the spinal cord, and to my knowledge there has been no convincing clinical trial in humans, otherwise this would be a mainstream therapy. Sorry, but I'm a natural skeptic and need to see the data before I'm willing to accept that what you say ("about 100% success") is correct. --- Medical geneticist (talk) 14:18, 6 November 2009 (UTC)
Stability of slopes
what is the application of stability of slopes —Preceding unsigned comment added by Nrnvgrao (talk • contribs) 03:24, 6 November 2009 (UTC)
- If Slope stability doesn't answer the question, you'll have to give more information. Looie496 (talk) 03:28, 6 November 2009 (UTC)
- See also "Angle of repose." Avoid slump. Edison (talk) 04:57, 6 November 2009 (UTC)
- This might be handy as well: Angle_of_repose -Craig Pemberton (talk) 05:03, 6 November 2009 (UTC)
Evolution of the hinged fang?
How could the solenoglyph's fang possibly have evolved? This strikes me as a really good poe question because it's hard to think of a functional intermediate. What do you guys think? -Craig Pemberton (talk) 04:55, 6 November 2009 (UTC)
- There's nothing particularly special about such a hinged tooth; other specialized teeth which seem equally striking, certainly exist. Indeed, the hinge itself doesn't involve any joints which do not have analogues in other vertebrates, its just that they evolved to bring the venom-delivering fangs into more efficient usage. One could easily envisage a series of progressively less-efficient, but still working, systems in ancestral species of such snakes. --Jayron32 07:14, 6 November 2009 (UTC)
- Yes you'd expect very strong evolutionary pressure to act with even quite slight improvements in teeth. They are pretty important in the 'survival of the fittest'! Dmcq (talk) 11:20, 6 November 2009 (UTC)
- (Especially in creatures with no claws or weapons of other kinds) SteveBaker (talk) 13:30, 6 November 2009 (UTC)
- Yes you'd expect very strong evolutionary pressure to act with even quite slight improvements in teeth. They are pretty important in the 'survival of the fittest'! Dmcq (talk) 11:20, 6 November 2009 (UTC)
- These hinged fangs have evolved more than once in snakes: "Folding fangs occur in two other groups of snakes. The Australian deathadders (Acantophis), though they are elapids, are solenoglyphous. Their folding-fang mechanism is very similar in appearance and operation to that of the vipers and pitvipers. The deathadders also have the body shape and ambush-hunting habits of many viperids, an excellent example of convergent evolution."[25]
- The hinged fangs are moved by palatal protractor and retractors muscles, the levator pterygoidei protractor pterygoidei and retractor pterygoidei pterygoideus respectively. These muscles attach further forward on a shortened maxilla in snakes with hinged fangs, details of the anatomy here:[26] In the evolution of 'venom delivery systems' in snakes, "dental glands became modified into Duvernoy's gland, posterior maxillary teeth became morphologically specialized relative to anterior maxillary teeth, and the anterior attachment of the pterygoideus muscle moved anteriorly, placing it in close association with the posterior maxillary teeth or fangs."[27] Basically, the development of fangs, hinges and venom just made innovative use of existing anatomy. Fences&Windows 19:17, 6 November 2009 (UTC)
Difference between airport and aerodrome
what is diffrent between airport and aerodrome? —Preceding unsigned comment added by 80.191.114.227 (talk) 07:03, 6 November 2009 (UTC) —Preceding unsigned
I have reformatted the question Richard Avery (talk) 07:12, 6 November 2009 (UTC)
- In the Canadian regulations [28], an aerodrome is "Any area of land, water (including the frozen surface thereof) or other supporting surface used, designed, prepared, equipped or set apart for use either in whole or in part for the arrival, departure, movement or servicing of aircraft and includes any buildings, installations and equipment situated thereon or associated therewith." An airport is an aerodrome certified to conform to certain regulations with respect to "obstruction surfaces, physical characteristics, marking and lighting, which have been recorded in an Airport Operations Manual, and Airside Operating Procedures." (from above link). So basically, all airports are aerodromes, but only some aerodromes are airports. (I believe this also is the same under international rules). -- Flyguy649 talk 08:26, 6 November 2009 (UTC)
- An airport - is a port - just like a shipping port - someplace where goods and people come in and out of a state or country. An airfield is some place where you can land and take off with aircraft - perhaps just for joyrides or local traffic. Generally, airports have customs and immigration facilities but airfields don't. Wiktionary says that in British English, an aerodrome has to have those things too so an aerodrome is an airport - but in Australian and Canadian English, it means the same thing as an airfield. I've never heard an American use the word and it's unclear what it means in American English. The word is pretty archaic though - I'd stick with "airfield" and "airport". SteveBaker (talk) 13:28, 6 November 2009 (UTC)
- In British English, aerodrome is somewhat archaic and little used. We would tend to use airport and airfield as above. --Phil Holmes (talk) 15:12, 6 November 2009 (UTC)
- Here in the USA, the only time I've heard the word "aerodrome" used seriously is to refer to Old_Rhinebeck_Aerodrome. APL (talk) 06:45, 7 November 2009 (UTC)
- Only an "international airport" would have customs and immigration, a ordinary domestic airport wouldn't. Rmhermen (talk) 14:40, 6 November 2009 (UTC)
strength of a C-C bond
From the carbon-nitrogen bond article: "The bond strength in a CN bond is higher (184 kcal/mol) than that of the CC bond (145 kcal/mol) [2]".
Is it talking about a C=C double bond? I thought the strength of a C-C bond was around 82 kcal/mol. John Riemann Soong (talk) 08:38, 6 November 2009 (UTC)
- I would remove the line from the article altogether, since it is highly a highly ambiguous statement. If you want to make an apples-to-apples comparison, see [29]. The data is in kJ rather than kcal, but it looks like a C-C bond is stronger than a C-N (though the C-N bond is shorter); however the C=C bond is weaker than the C=N bond, and the C≡C bond is weaker than the C≡N. I would use that source and fix the article to be less ambiguous. The source in the article NOW is simply the CRC Handbook, and while I have a copy of said Handbook, I'm not going to leaf through 1000 or so pages of tiny writing to try to find where that particular editor found that particular nugget and see what he really meant. I would just use the new, unambiguous source and fix the article. --Jayron32 16:49, 6 November 2009 (UTC)
Camphire in singapore
can you find camphire in singapore —Preceding unsigned comment added by 218.186.12.230 (talk) 10:09, 6 November 2009 (UTC)
- Camphire is (I believe) Lawsonia inermis - or more commonly Henna. Our article says "It is native to tropical and subtropical regions of Africa, southern Asia, and northern Australasia in semi-arid zones." - which suggests that it could probably be found in Singapore. It seems that Henna dye is used extensively in Singapore for tattoos - but, I couldn't find anything that definitely says that it grows there. SteveBaker (talk) 13:19, 6 November 2009 (UTC)
- Henna seems to grow in Malaysia,[30] like Steve I can find nothing about it growing in Singpore. Henna is sold and used in Singapore though. Fences&Windows 18:42, 6 November 2009 (UTC)
sulfuryl chloride: how does it replace a C-OH bond with a C-Cl bond?
Apparently this is what happens in the production of sucralose... I struggle to get a mechanism because all the bond energies seem disfavourable (C-O is stronger than C-Cl; O-H is stronger than O-Cl). In fact, most favourable seems to be chlorination at a hydrogen atom site ... I know it is by a free radical mechanism. What generally happens when a halogen and an alcohol group are on the same carbon atom? I get the feeling that one pushes the other out...John Riemann Soong (talk) 10:10, 6 November 2009 (UTC)
- The article Sulfuryl chloride does discuss the process, if not the mechanism, of sulfuryl chloride as a chlorinating agent. Since the molecule is itself somewhat unstable, and will over a relatively short time (days/weeks) spontaneously decompose into sulfur dioxide and chlorine I suspect that the reactive compound in this case is NOT the sulfuryl chloride, rather the actual reactive bit is diatomic chlorine; the sulfuryl chloride is merely a source of diatomic chlorine; being a liquid it is much easier to work with than a gas. Our article hints at this. Thus, it seems that the mechanism is a simple radical chlorination reaction; see Free radical halogenation. --Jayron32 16:42, 6 November 2009 (UTC)
π0
decay
When a pion decays into 2 photons by
π0
→ 2
γ
what is the energy in eV of the resulting photons?--IngerAlHaosului (talk) 10:36, 6 November 2009 (UTC)
- In the rest frame of the pion, conservation of energy tells you that the total energy of the photons must be equivalent to the rest mass of the pion, and conservation of momentum tells you that the photons must have equal but opposite momentum vectors, therefore equal wavelengths and therefore equal energies. I'll let you take it from there. Gandalf61 (talk) 14:15, 6 November 2009 (UTC)
Fluid mechanics
What is the use of coefficent of discharge,coefficent of velocity and contraction? —Preceding unsigned comment added by Nrnvgrao (talk • contribs) 11:13, 6 November 2009 (UTC)
- These are empirical factors to account for the first-order deviation between theoretical and actual values of the discharge, velocity, and contraction of a fluid. See flow coefficient. Nimur (talk) 13:51, 6 November 2009 (UTC)
GRAVITATIONAL RED/BLUE SHIFT
210.212.239.181 (talk) 12:43, 6 November 2009 (UTC)HARSHAGG
ALSO is there gravitational blue shift. Now consider light coming out perpendicular to surface of earth then graviton moving at c can't interact with light so no red shift can observe. Is this feasible because I can't find solution to this they just use classical concept of potential energy(classical in sense the one i know if there exist another concept i am not aware of
210.212.239.181 (talk) 12:38, 6 November 2009 (UTC)HARSHAGG HI I was reading the page on black hole and came across that when charge say +ve go inside black hole then applying gauss law there should be electric field but no photon can come out of black hole so no electromagnetic interaction can be there but it happens. This question is unanswered I contact my teacher they also don't know about this.
- We have an introductory article on charged black holes. Needless to say, the mathematics to describe the behavior is extremely complicated; there is a quick description of the double event horizon. It is speculated (by prominent scientists in the field) that electrostatic repulsion would prevent such a charged black hole from forming naturally. Nimur (talk) 13:57, 6 November 2009 (UTC)
- The electromagnetic field of a charged black hole comes from the charged material that collapsed to form the black hole, before it crossed the event horizon. It doesn't come from beyond the event horizon—any change in the distribution of charge inside the event horizon has no effect on the field outside. The same is true of the gravitational field. These are sometimes called "fossil" or "relic" fields. If you're wondering why the field doesn't redshift out of detectability, well, it turns out that fields don't work that way. I don't truly understand why.
- I don't know how to explain this in terms of virtual particles. Probably it's a bad idea to think about it in those terms. The theory objectively predicts that nothing that happens inside the event horizon affects the field outside, so any explanation that claims that "virtual particles can escape the event horizon" will have to ensure that those virtual particles don't have any observable effect on the outside, so they might as well not have escaped the event horizon at all. -- BenRG (talk) 16:32, 6 November 2009 (UTC)
Infinite differential method
what is the use of infinite differntial method in solving problems in physics? —Preceding unsigned comment added by Snehrajravi (talk • contribs) 16:59, 6 November 2009 (UTC)
Bird bones
I have two bird bones. They are a left and a right. They are not long bones. I do not know what they are! They look sort of like two tiny femurs with broad, triangular bases and a prong sticking out just above the broad base. One of the points of this base has a round hole in it. They aren't in my avian osteology book and I can't find them through googling pictures of bird bones. Help? 138.192.58.227 (talk) 17:13, 6 November 2009 (UTC)
- Identification would be easier if you could photograph them and upload the photos to a Flickr or Photobucket account, and link to the photos from here. Comet Tuttle (talk) 18:38, 6 November 2009 (UTC)
- Yeah -- definitely take a photo. There is already a lack of proper communication, as you state they appear as 'femurs' yet assert they 'are not long bones.' The femur is a long bone, and if you were using 'long' as a mere adjective (and not referring to the aforementioned link), how long do you expect a bird's bone to be anyway...sort of subjective to the extreme. DRosenbach (Talk | Contribs) 19:56, 6 November 2009 (UTC)
- How do you know they're bird bones? Assuming that they are, and that they don't look like what's in your textbook, the thing to consider is how easily bones can be shaped to suit different circumstances (i.e. adaptive radiation). Examine the bones and try to identify points of attachment, etc. and look through your book for bones which have the same kind of pattern, if not necessarily the same shape. The femur of a crow, for example, will look wildly different from the femur of a heron in terms of shape, but the basic pattern of structures will largely be the same. Matt Deres (talk) 04:38, 8 November 2009 (UTC)
Emission spectra
I understand which wavelengths can be emitted by a given element, but not where you can calculate (rather than observe) the percentage of each type of photon emitted. Clearly, you can observe this, and I would imagine it is governed by a probability system. Have we (that is, the scientific community) worked this out yet? In short, can you calculate the total colour (i.e. the apparent colour) of a given element, not just which frequencies it's made up of? - Jarry1250 [Humorous? Discuss.] 17:17, 6 November 2009 (UTC)
- I'm not sure what you mean. The only way you could calculate the wavelengths of light emitted by an atom absent of making any measurements is via the Rydberg formula; however that only applies to the Bohr model atoms, i.e. 1-electron atoms (H, He+1, Li+2, etc.) For any atom with more than one electron, you have an n-body problem, and no simple algebraic function can be written to predict the wavelengths emitted by such atoms; you can only observe the wavelengths, not mathematically predict them as with the Rydberg formula. --Jayron32 18:35, 6 November 2009 (UTC)
- I think the question presumes that you already know the wavelengths of an element's emission lines. How do you determine the relative strength of each line (and hence the visual appearance of the emission)? The answer will depend on various factors (temperature, density, incident radiation). How well can we predict the line strengths given the circumstances? -- Coneslayer (talk) 19:34, 6 November 2009 (UTC)
- Color is a complex phenomena that is hard to quantify; there are many methods of plotting color in various multiple dimensions, for example. While we can quantify a single wavelength of light as a certain value, to "quantify" a bulk color made up of many wavelengths with a series of values is a difficult thing to do. Color is really about perception and not quantification. Consider, for example, that the color we call "yellow" could be made of a single wavelength OR it could be made of mixture of multiple wavelengths of light, and yet our mind would find two such colors indistinguishable; that is our color perception equipment in our minds cannot distinguish between coherant light of a single wavelength or a color made of an average of wavelengths.
- As far as determining the bulk color of an excited substance; that's easy. Just look at the color before the light is passed through a prism. Emission spectra still require a prism or diffraction grating to seperate the wavelengths into an actual spectrum, so if you want to know what color such a spectrum would make if mixed together, just look at the light before it passes through the prism! --Jayron32 19:58, 6 November 2009 (UTC)
- You're talking about observations, which the OP is specifically not interested in. He's asking about predicting the emission spectrum (line strengths, not just wavelengths) from physical principles. -- Coneslayer (talk) 20:04, 6 November 2009 (UTC)
- See below by Coneslayer. That's the whole point, for atoms with more than one electron, you have an n-body problem which is almost imposible to predict via mathematics. It's been done for hydrogen and helium, and those required some rather complex modeling and some serious computing power. Once you get to atoms like, say, Carbon, with multiple electrons in multiple ground state orbitals, the system is just way too chaotic to even attempt a prediction. We can observe the light coming off, but we cannot predict a priori knowing only the ground state energy levels, what anything about the spectrum (wavelength OR intensity) --Jayron32 02:57, 7 November 2009 (UTC)
- Thanks for the pointer. That Coneslayer guy is pretty sharp. -- Coneslayer (talk) 04:00, 7 November 2009 (UTC)
- Here is one code to model emission spectra for astrophysical sources: CLOUDY. The references in the "Predicted intensities of hydrogen and helium lines" section of the FAQ may be helpful. I think the short answer is that even for relatively simple atoms, "it's hard". -- Coneslayer (talk) 20:10, 6 November 2009 (UTC)
(reply to all). Thanks. Coneslayer is right about what I meant, and your answers are very helpful. - Jarry1250 [Humorous? Discuss.] 11:38, 7 November 2009 (UTC)
5-membered dioxane formation from a sugar
Help! I don't know how a diol on a straight-chain sugar is supposed to form a 5-membered heteroatom ring ... basically the alcohol oxygens are geminal ethoxy atoms on a propyl chain, (but vicinal diols on the sugar chain) ... John Riemann Soong (talk) 18:22, 6 November 2009 (UTC)
- Ring closure in sugars is a type of hemiacetal or hemiketal reaction, see also Furanose which is the 5-membered ring of which you speak. --Jayron32 18:28, 6 November 2009 (UTC)
- Ummm, how do I put this protecting group on? Googling is so frustrating! It's not a ring closure via the carbonyl -- it's an extra protecting group that gets put on the secondary alcohols; the carbonyl and the primary alcohols remain untouched. John Riemann Soong (talk) 18:31, 6 November 2009 (UTC)
- It's also a dioxane ... that is there are two ethoxy oxygens in this ring. The sugar backbone is straight-chained. HELP! I'm going to die in 3 hours! John Riemann Soong (talk) 18:33, 6 November 2009 (UTC)
- You're going to have to provide more details. Do you have the complete problem you are trying to solve? I cannot help you without more information. If this is a homework problem (like a total synthesis problem) or something, we're going to need the exact wording so we can steer you in the right direction. Full structures and stuff would also be helpful. --Jayron32 18:37, 6 November 2009 (UTC)
- Apparently it's called an acetonide --- but all we have on the subject on acetonides is how they're used as drugs?!! John Riemann Soong (talk) 18:38, 6 November 2009 (UTC)
Here it is ... I have to start with a sugar. Apparently I have to put these protecting groups on ... then I can oxidise the alcohols and ketones into carboxylic acids (or acid chlorides...?) The problem is that the protecting groups are sensitive to acid so acid-catalysed esterification after that might be an issue. John Riemann Soong (talk) 18:41, 6 November 2009 (UTC)
- The protecting groups in this case are acetone ketals. Normally, you protect a ketone by using something like Ethylene glycol to form the cyclic ketal; however the reverse is perfectly valid, you can do a protection of vicinal diols by using a simple ketone, like acetone. That is all that is done here. Look up the mechanism of ketal protection of ketones, and its the exact same mechanism for forming the groups in that molecule. It's basically a ketal protection in reverse.--Jayron32 18:48, 6 November 2009 (UTC)
- I really don't get how this protection works. Do you basically kick out the carbonyl oxygen and form a carbocation that the other diol can bind to? :S John Riemann Soong (talk) 18:52, 6 November 2009 (UTC)
- Yes, the second OR replaces the OH (that OH being the carbonyl oxygen atom) by an SN1 reaction. DMacks (talk) 18:55, 6 November 2009 (UTC)
- I really don't get how this protection works. Do you basically kick out the carbonyl oxygen and form a carbocation that the other diol can bind to? :S John Riemann Soong (talk) 18:52, 6 November 2009 (UTC)
- Also, should I put this protecting group on before I do oxidation of the primary alcohols / aldehydes...? John Riemann Soong (talk) 18:59, 6 November 2009 (UTC)
- See this document and page down till you get to the section titled "MECHANISM FOR THE ACID catalyzed FORMATION OF ACETALS" It contains the full electron-pushing mechanism you seek. In YOUR case, instead of using two different ethanol molecules to form the acetal, you would be using the neighboring vicinal -OH groups in the sugar, but it is otherwise identical to that mechanism. --Jayron32 19:08, 6 November 2009 (UTC)
Age Re-Perfect Pro-Calcium L'Oréal Paris
What does "Pro-Calcium" means? I know there are some substances with the prefix "pro", but does it make any sense here? Quest09 (talk) 18:52, 6 November 2009 (UTC)
- Yes, "pro-calcium" is a perfectly valid marketting term, regardless of whether there is any actual factual/scientific meaning behind it or whether or not that meaning is relevant to the context and intended purpose. DMacks (talk) 18:56, 6 November 2009 (UTC)
- I suppose there is no meaning behind that, but I am not completely sure. Anyway, I don't know if it is perfectly legitime to use the name. If the intention is to make people believe the product has some advantage that it doesn't - like fixating calcium - you could have trespassed a moral barrier, even if legally it is allowed.--81.47.159.223 (talk) 19:53, 6 November 2009 (UTC)
- The safest position, in the absence of any other evidence, is to assume that the marketing terms used by cosmetics manufactures are pseudo-scientific bullshit. —Preceding unsigned comment added by 86.134.115.178 (talk) 00:58, 7 November 2009 (UTC)
- According to Wiktionary, we have five meanings for 'pro-' to choose between:
- agreeing with; supporting; favoring
- substituting for
- earlier; prior
- rudimentary
- in front of
- Something that 'supports' calcium is, I suppose, possible - maybe something that aided calcium uptake perhaps? Substituting for is an unlikely thing - if your body needs actual calcium, there is no substitute! Earlier than calcium?? That makes no sense. Rudimentary calcium - would be just calcium - you don't get much more rudimentary than a chemical element! In front of calcium...again, makes no sense.
- So the only meaning that really makes sense is that this is something that helps calcium do it's job in some way. However, as others have correctly pointed out, cosmetics manufacturers appear to feel absolutely ZERO need to make any sense whatever - so this could mean anything. Also, this is "Pro-Calcium(tm)" - they couldn't have trademarked the name of a real chemical element - so this is evidently something they just thought up.
- Loreal's own web page says that it's made of "Calcium microspheres"...but such a thing would be incredibly reactive with water - definitely not something you'd want anywhere near your face. Most likely, it's calcium carbonate - which would be chalk dust.
- SteveBaker (talk) 01:55, 7 November 2009 (UTC)
- L'Oreal's "Pro-Calcium" products contain calcium pantetheine sulfonate, which in vitro, anyway, reduces the amount of pigmentation in skin cells.[31] The Pro-Calcium products also contain hydroxyapatite, a calcium-containing mineral which theoretically might improve your skin's barrier functions, although I can't readily find a study that would support that claim. See [32]. Red Act (talk) 02:07, 7 November 2009 (UTC)
Buteyko method
Is the Buteyko method woo-woo? -Craig Pemberton (talk) 19:30, 6 November 2009 (UTC)
- It does not sound like complete bullshit. Reading the article, it is basically physical therapy for asthma; that is training the breathing airways to remain open via deliberate intentional conditioning. Such methods sound plausible; however the article at Wikipedia also notes that the method requires dedication and committment on the part of the patient; so it may be possible that the method may not have as good of an outcome across a wide spectrum of patients, especially since some patients may not "do it right", and thus it may not work for them. The article unfortunately makes the method SOUND like bullshit, even if it is scientifically valid, since it uses such known bullshit terms like "holistic" and the phrase "There are no known negative trials" is worrysome because it does not define a "negative trial"; and I would not recognize that term in any actual scientific study anyways. So, on the face it looks like a plausible treatment for asthma, but that doesn't necessarily mean it is the best treatment for asthma. --Jayron32 19:50, 6 November 2009 (UTC)
- That's the thing: it begs to be called out, but I don't see any obvious flaws. For example, these two videos are eerily similar in format: utter bunk and a Buteyko seminar. Both are overly confident, cite a bunch of science they don't understand, ask the audience leading questions, etc. I'm just worried that the articles on stuff like Bohr effect seem legit only because they've slipped by the notice of most Wikipedians. -Craig Pemberton (talk) 20:33, 6 November 2009 (UTC)
- After reading this discussion, I went and did some minor clean-up to the article. Nowhere but the lead mentions "holistic" and "philosophy". One can't prove a negative so I removed the un-citable sentence about "no known negative trials". =Axlq 05:32, 7 November 2009 (UTC)
Killing Viruses
We have drugs that are capable of killing fungi and bacteria pathogens, however we do not have drugs that can kill viruses. Is this because any drugs that are capable of killing viruses are also capable of killing surrounding tissue? I checked the article on nanomedicine, and it didn't mention much about using nanomachines to kill viruses. Are there any ideas to use nanomachines to kill viruses? ScienceApe (talk) 19:51, 6 November 2009 (UTC)
- It's because viruses are technically not living. We have drugs that inactivate many of the processes they direct or undergo, such as antiretrovirals and protease inhibitors. And in reference to your comment about antifungals -- it's those drugs that are dangerous to surrounding human tissues, because fungi are eukaryotes and have much more similar (or identical) processes and components to humans and animals than do prokaryotic bacteria. For the most part, antifungals work to inhibit the fungi cell wall production, with many of them targeting ergosterol. Check out amphotericin B and it's terrible effects on the human kidney (hence the knickname 'amphoterrible B'). DRosenbach (Talk | Contribs) 19:57, 6 November 2009 (UTC)
- I'd dispute that. Viruses are not cellular life, but that is a rather restrictive view of life. Viruses replicate, usually but not necessarily with the help of others, inherit genetic mutations, and are subject to natural selection. You can't say the same about, say, fire. Imagine Reason (talk) 16:22, 7 November 2009 (UTC)
- You can dispute it all you want, but that's the biological classification. DRosenbach (Talk | Contribs) 03:28, 8 November 2009 (UTC)
- Really? Last I heard, it was still a largely disputed concept. It doesn't really matter, though - viruses are what they are whether we call them "life" or not. It's not that we don't understand what viruses are so we're not sure how to classify them, it's just about the definition of the word "alive". --Tango (talk) 04:01, 8 November 2009 (UTC)
- The definition of life is about as meaningful to me as the definition of a planet. It's subjected to mostly arbitrary rules and criteria so it's not really that important. Almost certainly the definition of life would have to change if we discover life on other planets anyway, or if the origin of life is finally determined. The origin of life is still an unknown so no one knows what life really is. ScienceApe (talk) 05:19, 8 November 2009 (UTC)
- Really? Last I heard, it was still a largely disputed concept. It doesn't really matter, though - viruses are what they are whether we call them "life" or not. It's not that we don't understand what viruses are so we're not sure how to classify them, it's just about the definition of the word "alive". --Tango (talk) 04:01, 8 November 2009 (UTC)
- You can dispute it all you want, but that's the biological classification. DRosenbach (Talk | Contribs) 03:28, 8 November 2009 (UTC)
- I'd dispute that. Viruses are not cellular life, but that is a rather restrictive view of life. Viruses replicate, usually but not necessarily with the help of others, inherit genetic mutations, and are subject to natural selection. You can't say the same about, say, fire. Imagine Reason (talk) 16:22, 7 November 2009 (UTC)
- As for nanomachines - we don't really have any of those that are useful for much yet. The state of the research is far to early along a very long development trail for them to be useful for anything much yet. Nanomaterials are starting to become very useful though - so we'll probably get there in the end. So for now we're stuck with much more conventional medicine. Part of the difficulty here is that for some parts of the viral life-cycle, they are nothing more than little bits of DNA that are floating around in our cells tricking them into making more virusses. At that phase in that cycle, they are inside the cell, looking and behaving just like any other chunk of DNA. That's gonna make them exceedingly hard to destroy. So while you might come up with some scheme to remove all of the virusses floating around outside of the cells - there would always be some small percentage that would be tucked away, almost impossible to reach. SteveBaker (talk) 01:36, 7 November 2009 (UTC)
- Nanotech would struggle to directly "kill" a virus. According to our article, virus, viruses are typically between 10 and 300nm in diameter. That makes them about the same size as nanotech. The difficulty of doing anything directly to viruses is why we use things like the protease inhibitors mentioned above - they stop the infected cells producing more viruses rather than doing anything to the viruses themselves. --Tango (talk) 01:45, 7 November 2009 (UTC)
- Doesn't our immune system destroy viruses? How do they destroy viruses? ScienceApe (talk) 03:41, 7 November 2009 (UTC)
- Things like Cytotoxic T cells will kill cells infected by viruses. I'm not sure the immune system does anything to directly kill viruses. --Tango (talk) 04:45, 7 November 2009 (UTC)
- Also, things like antibodies or immunoglobins are used by the body to tag viruses for cleanup by other methods, but this again doesn't "destroy" the virus, it merely identifies the virus for cleanup by T-cells. --Jayron32 05:34, 7 November 2009 (UTC)
- What does cleanup by T-cells mean exactly? How does it clean it up? ScienceApe (talk) 18:23, 7 November 2009 (UTC)
- See my link - the T-cells kill the cell containing the virus, thus preventing the virus using that cell to reproduce. --Tango (talk) 19:30, 7 November 2009 (UTC)
- I know I already looked at it. I thought he meant something else by "clean it up" since you already mentioned that the T-cells kill infected cells. ScienceApe (talk) 20:32, 7 November 2009 (UTC)
- Ah, I don't think so. I think he was just explaining that there is more to the immune system's response to viruses than the T-cells I mentioned, and he's absolutely right. --Tango (talk) 20:45, 7 November 2009 (UTC)
- I know I already looked at it. I thought he meant something else by "clean it up" since you already mentioned that the T-cells kill infected cells. ScienceApe (talk) 20:32, 7 November 2009 (UTC)
- See my link - the T-cells kill the cell containing the virus, thus preventing the virus using that cell to reproduce. --Tango (talk) 19:30, 7 November 2009 (UTC)
- What does cleanup by T-cells mean exactly? How does it clean it up? ScienceApe (talk) 18:23, 7 November 2009 (UTC)
- Also, things like antibodies or immunoglobins are used by the body to tag viruses for cleanup by other methods, but this again doesn't "destroy" the virus, it merely identifies the virus for cleanup by T-cells. --Jayron32 05:34, 7 November 2009 (UTC)
- Things like Cytotoxic T cells will kill cells infected by viruses. I'm not sure the immune system does anything to directly kill viruses. --Tango (talk) 04:45, 7 November 2009 (UTC)
- Doesn't our immune system destroy viruses? How do they destroy viruses? ScienceApe (talk) 03:41, 7 November 2009 (UTC)
- Nanotech would struggle to directly "kill" a virus. According to our article, virus, viruses are typically between 10 and 300nm in diameter. That makes them about the same size as nanotech. The difficulty of doing anything directly to viruses is why we use things like the protease inhibitors mentioned above - they stop the infected cells producing more viruses rather than doing anything to the viruses themselves. --Tango (talk) 01:45, 7 November 2009 (UTC)
- And, since no one seems to have bothered, we do have an article, or rather subsection, on this, see Virus#Host defence mechanisms
effeminacy
What is the medical term for males who are effeminate but do not desire to have sex with other males but rather with females? 71.100.0.254 (talk) 20:22, 6 November 2009 (UTC)
- That doesn't sound like a medical condition. Why do you presume that there should be a medical term for it? -- Coneslayer (talk) 20:35, 6 November 2009 (UTC)
- This isn't considered a medical condition. You could call such people "effeminate heterosexual males" if you were obsessed with the taxonomy of gender and sexuality. Hey, Gender taxonomy is an article on Wikipedia, though it seems to be mostly about hermaphrodites and not what the OP was asking. Comet Tuttle (talk) 20:50, 6 November 2009 (UTC)
- It isn't the same thing but some reference from metrosexual might get what you want. Dmcq (talk) 20:57, 6 November 2009 (UTC)
- Yes, that is what I was thinking. --Mr.98 (talk) 21:59, 6 November 2009 (UTC)
- It may be a medical condition, some type of intersexuality. Rmhermen (talk) 23:43, 6 November 2009 (UTC)
- Yes, that is what I was thinking. --Mr.98 (talk) 21:59, 6 November 2009 (UTC)
- There may be evolutionary advantages in harem species for the effeminate males to sneak around the dominant males. Imagine Reason (talk) 23:55, 6 November 2009 (UTC)
- I seem to recall that a species of cuttlefish does something like that. While the big males are fighting to figure out who gets the female, a scrawny male changes his color to look like a female, slips past them, mates with the female, then skedaddles before they catch on. StuRat (talk) 06:38, 7 November 2009 (UTC)
- Interesting idea, but if this success happens with any regularity wouldn't it alter the male cuttlefishes' mating behaviour. The successful male would pass on, through his genes, the successful strategy. Richard Avery (talk) 11:13, 7 November 2009 (UTC)
- There seems to be a limiting factor: If too many scrawny males use this strategy, then the big males catch on and watch for this, and the "effeminate" strategy no longer works. StuRat (talk) 13:45, 7 November 2009 (UTC)
- I've had friends who are sometimes mistaken for gay because of their perceived effeminacy - what they seem to have in common is that all have been brought up by their mothers - I presume their epicenity stems from the fact that their primary role model was female. Adambrowne666 (talk) 13:20, 7 November 2009 (UTC)
Floor Stain (help!)
A couple nights ago I dropped my Drinking Bird, the glass broke and the contents ( red dye in Dichloromethane ) splashed on my kitchen floor which I assume to be made of Linoleum. I have been unsuccessul in removing the stains, having tried various caustic cleaners, petroleum based removers and even 30% Hydrogen peroxide. Is using pure Dichloromethane my only hope? I know the chemical has probably melted or fused itself with the plastic floor. Thanks for any suggestions. cheers, 10draftsdeep (talk) 20:29, 6 November 2009 (UTC)
- Our article says it is miscible with many organic solvents, so the petroleum based removers were probably along the right lines. Have you tried things like white spirit or acetone (nail varnish remover)? --Tango (talk) 20:49, 6 November 2009 (UTC)
Not yet. Thanks, I'll try some acetone.10draftsdeep (talk) 20:58, 6 November 2009 (UTC)
- Be careful, though: organic solvents (such as acetone) will dissolve plastics. Test whatever you're using on an out-of-the-way corner first. --Carnildo (talk) 21:42, 6 November 2009 (UTC)
- Indeed - that applies to any cleaner, but I should probably have said it anyway. --Tango (talk) 22:08, 6 November 2009 (UTC)
Will do. Although it appears the Dichloromethane has already removed the top layer of the floor's surface where it landed. Ultimately ,I am starting to think some new flooring may be the final solution.10draftsdeep (talk) 21:49, 6 November 2009 (UTC)
- Also, if you're planning on using large quantities of acetone, be sure the area is well-ventilated. If it's cold where you are, wait until the hottest part of the day and put a coat on, then open some windows and use the acetone. StuRat (talk) 06:35, 7 November 2009 (UTC)
calculating the pH of a solution with a polyprotic amino acid
Let's say I have an amino acid (not a biological one -- say it's 4-aminobenzoic acid with two pKa's: 2.50 at the +1 state and 4.87 in the +0 state... let's say the concentration is 0.1M. How do I calculate what the pH of the solution will be? Do I use the isoelectric point? John Riemann Soong (talk) 20:44, 6 November 2009 (UTC)
I'm trying to come up with an equation ... can someone help me. So basically the neutral compound can be deprotonated ... and protonated.
I get stuck around here. I'm trying to some "proton accouting" equations, but I can't wrap my head around it. It would be really nice if there were some way to determine the ratio of AH2+ to A-! John Riemann Soong (talk) 00:08, 7 November 2009 (UTC)
With some further rearranging, I get equations like:
(0.1 is my concentration of my reagent.)
Is an equation like A- + H+ = 0.1 valid here? John Riemann Soong (talk) 00:21, 7 November 2009 (UTC)
November 7
Name that chemical
When I started working here in the semi-conductor manufacturing business, I was told of a chemical that wouldn't harm skin if the skin came in contact with the chemical. What would happen though, is that the chemical would soak into the tissue and settle in the person's bones and that's where it would cause considerable pain. I've looked it up before but it's been years. Can anyone remind me what this chemical is? Dismas|(talk) 05:58, 7 November 2009 (UTC)
- Sounds a lot like benzene. Benzene causes bone marrow damage, can be absorbed through the skin,[33] and is used in the semiconductor industry.[34] Red Act (talk) 06:31, 7 November 2009 (UTC)
- Thanks for the info about benzene but hydrofluoric was the one I was thinking of. Thanks, Dismas|(talk) 11:35, 7 November 2009 (UTC)
is it possible I received permament eye damage from briefly handling apple's laser mouse at an Apple store.
Hi, this is not a request for medical advice, I'm asking if something is scientifically possible. I saw the advertisement for Apple's laser mouse (magic mouse) among other things a while ago, and today was near an apple store and tried one out. During this time I just moved it around, tried scrolling, and I also looked at the bototm of it basically twice, as I remembered from the advertisements that there was a switch on the very bottom of it (http://www.gizmag.com/pictures/gallery/apple-magic-mouse-3.jpg) and I wondered what it does. I switched it, used the moues a little more, couldn't see a difference, then switched it back and left the store. I doubt I looked at the back of the moues for more than a few seconds total, certainly not a prolonged period of time. Here's the thing though: basically within minutes of leaving the store I started noticing a rectangular after-effect in the center of my vision when I blinked, it was immediately after I left the store that I noticed it, so I started thinking about that laser mouse immediately. Now it's the next morning, this is like 18 hours later and I still see it. Is it possible I got permament eye damage from just briefly handling a laser mouse? Normally I would think it's totally out of the question for this to be a possibility with such a product, but this was a display item, who knows, maybe it was damaged or something. Anyway I'm just asking for the possibility of the explanation for what I'm seeing. I'm not asking for medical advice. Just physical possibility. Thank you. 92.230.66.64 (talk) 07:47, 7 November 2009 (UTC)
- this gives me a bit of relief though: http://web.princeton.edu/sites/ehs/LabPage/laserpointersafety.htm "Afterimage is the perception of spots in the field of vision. This can be distracting and annoying, and may last several minutes, although there have been reports of afterimages lasting several days.". "Several individuals have reported temporary blindness when targeted by a number of laser pointers. This is becoming more prevalent at sporting events. A few individuals complained of afterimages lasting several days." I think it would be mentioned if it were permament. phew! —Preceding unsigned comment added by 92.230.66.64 (talk) 08:00, 7 November 2009 (UTC)
Any sudden, persistent change in your vision is reason to seek medical advice. The only medical advice we can give here is to consult a doctor. I think you should consult a doctor.
- Various medical conditions could cause a sudden visual change. An opthalmologist could test vision and examine the retina. Edison (talk) 04:06, 8 November 2009 (UTC)
--Anonymous, 09:21 UTC, November 7, 2009.
- Thank you for understanding that we cannot give medical advice, OP, and making an attempt to find out the answer yourself about whether it is a physical possibility. --KageTora - SPQW - (影虎) (talk) 10:48, 7 November 2009 (UTC)
- The Magic Mouse is a Class 1 Laser so, in theory, it should be pretty safe, even if you stare into all day (not recommended). That being said, as others have reiterated, if you have a sudden change in vision, definitely schedule an eye appointment—if it goes away on its own before then, you can always cancel it! If not, you'll be glad you have done it. --Mr.98 (talk) 12:41, 7 November 2009 (UTC)
- Saying "I'm not asking for medical advice" doesn't make it so. You are asking for medical advice. Go and see a doctor. --Tango (talk) 18:00, 7 November 2009 (UTC)
To answer the hypothetical. It is improbable that the laser pointer in a mouse could cause significant or lasting damage to a healthy eye. There just isn't much power there. Further, it should be impossible to damage the eye with a visible laser and not immediately realize that one had looked at a laser. The experience would be immediately blinding and potentially painful. So, I would say the mouse is very unlikely to be the cause of the problem you describe. Now, let me be very clear, any sudden, persistent change is a reason to see a doctor. I am telling you it is not the mouse, in part because I think it is important you not dismiss your symptoms for that reason. Go talk to a real doctor. Dragons flight (talk) 20:06, 7 November 2009 (UTC)
Taxonomy
Could please someone provide the taxonomic name of these flowers? Thanks in advance! -- Etienne (talk) 13:55, 7 November 2009 (UTC)
- Wow, a botany question that I can actually answer -- the first time ever! The first one looks like common madia, better known as tarweed. The picture in the common madia article doesn't look the same, but I think I'm right, it's a pretty common flower here in the Bay Area. (If not common madia, then some other type of madia, there are a ton of them.) I don't know what the second one is. Looie496 (talk) 17:31, 7 November 2009 (UTC)
- The second one is cosmos_bipinnatus, I also have some doubts that the first is common madia. Richard Avery (talk) 18:44, 7 November 2009 (UTC)
Is lightning a spark?
Arguing definition is fun! Vimescarrot (talk) 15:59, 7 November 2009 (UTC)
Slow-motion footage of lightning (Please don't embed 2MB animated GIFs to the Ref Desk... some of us don't have the fastest connections and it screws up the whole page. Changed from embedded image to link... --Mr.98 (talk) 18:13, 7 November 2009 (UTC).)
- As fun as it may be, the ref desk is not a chat room or a place for debates. Though I don't see what there is to argue about: see Electrostatic_discharge#Sparks. —Akrabbimtalk 16:26, 7 November 2009 (UTC)
- Yes. Both "lightning" and "sparks" are essentially electrostatic discharges. The only significant difference is perhaps that of intensity: lightning is actually an electric arc, while a "spark" technically need not "plasma-ize" the air it passes through (apparently, at least according to the intro of the Corona discharge article...).
- In any case, according to Electrostatic discharge#Sparks:"Perhaps the best known example of a natural spark is a lightning strike."
- Wikiscient 16:36, 7 November 2009 (UTC)
- I saw the discharge article, but wanted to check with you to make sure the article was right. Sorry, I didn't mean to imply I wanted to spark (ha!) an argument with you; I'm arguing with someone else. Thanks for the responses. Vimescarrot (talk) 16:43, 7 November 2009 (UTC)
- Well in that case, argue your heart out! —Akrabbimtalk 16:49, 7 November 2009 (UTC)
- I think the best way to look at it is: lightning is a "spark," but not all sparks are lightning... ;) Wikiscient 16:51, 7 November 2009 (UTC)
- I saw the discharge article, but wanted to check with you to make sure the article was right. Sorry, I didn't mean to imply I wanted to spark (ha!) an argument with you; I'm arguing with someone else. Thanks for the responses. Vimescarrot (talk) 16:43, 7 November 2009 (UTC)
- That animated GIF of the lightning is fake, right? Tempshill (talk) 19:42, 7 November 2009 (UTC)
- I think it's real. And how dare people not wait for me to get to this question. SpinningSpark 21:47, 7 November 2009 (UTC)
- I would love to know the detail of how it was filmed. How fast a camera is being used there? APL (talk) 00:03, 8 November 2009 (UTC)
- Don't know about that gif, but Ultraslo uses 3000 FPS. That's not the only slow-motion lightning video on Youtube, and many put their FPS in their description. Vimescarrot (talk) 00:31, 8 November 2009 (UTC)
- We used cameras (and camera-like devices) with frame rates as high as 15,000 frames per second, to record lightning and lightning-related transient luminous events. Often, these high-data-rate optical devices needed electronic triggers to activate them and initiate a burst-mode recording. Nimur (talk) 03:57, 8 November 2009 (UTC)
- Ben Franklin's and others' research in the 1750's indicates an affirmative answer. Edison (talk) 04:04, 8 November 2009 (UTC)
Torch lights
Will shining 2 torch lights into a particular area make the resulting light brighter? Clover345 (talk) 17:37, 7 November 2009 (UTC)
- Yes. It won't, however, make it look twice as bright. The human eye perceives brightness logarithmicly, that means doubling the actual brightness (by shining twice as much light at it) will make it look a fixed amount brighter (ie. it will add something to the brightness rather than multiply the brightness by 2). See Weber–Fechner law#The case of vision. --Tango (talk) 18:05, 7 November 2009 (UTC)
- Also, don't forget interference (as shown by Young's slit experiment. Shutting off a light in the room will lower the overall amount of light in the room, but the areas that were previously in the interference path of multiple light sources might actually get brighter. John Riemann Soong (talk) 23:11, 7 November 2009 (UTC)
- That's a really terrible answer! Do you have any idea how narrow those interference regions are? No! Of course you don't or you'd never have said that. You'd need a microscope to see them. Where are these "slits" that are necessary for the effect to work? Do you realize that flashlight bulbs or flashlight LED's are not point sources - and they aren't monochromatic and they aren't coherent? Have you ever heard of diffuse scattering? A little knowledge is a dangerous thing! It is absolutely not the case that turning on a second flashlight will make any measurable part of the room measurably darker. Your answer is confusing and flat out incorrect. Tango had it right - the end. SteveBaker (talk) 23:48, 7 November 2009 (UTC)
Why do my glasses have a shadow?
My eyeglasses are transparent and yet when it's very bright and light is angled properly, the lenses have a shadow. Why is that? 69.77.250.210 (talk) 19:13, 7 November 2009 (UTC)
- Lenses work by difraction, bending and redirecting the incident light. There is still the same amount of light hitting the lens, but because it is not going "straight through", the area straight through is not as brightly illuminated as the surrounding area that is directly illuminated. DMacks (talk) 19:26, 7 November 2009 (UTC)
- It could also be because they aren't perfectly transparent. The diffraction (note spelling!) can cause some bright spots which are brighter than the surrounding area, this means the average brightness is about the same. --Tango (talk) 19:29, 7 November 2009 (UTC)
- Thank goodness redirects help when my riting is not good. DMacks (talk) 19:32, 7 November 2009 (UTC)
- Yes, diffraction is the answer. See also Lens (optics) and Caustic (optics). Red Act (talk) 19:45, 7 November 2009 (UTC)
- My eyeglasses work by refraction, not diffraction. -- Coneslayer (talk) 20:10, 7 November 2009 (UTC)
- It could also be because they aren't perfectly transparent. The diffraction (note spelling!) can cause some bright spots which are brighter than the surrounding area, this means the average brightness is about the same. --Tango (talk) 19:29, 7 November 2009 (UTC)
- Note as well that even in the absence of diffraction effects your lenses aren't perfectly transparent, and they do not transmit all of the light that strikes them. Depending on the material the lenses are made from, and what coatings have been applied, somewhere in the neighbourhood of 10% of the incident light is going to be reflected or absorbed. TenOfAllTrades(talk) 19:51, 7 November 2009 (UTC)
- As far as I know no corrective glasses work by diffraction but rather refraction. I'm assuming the OP has myopia as my glasses do the same thing too. If you try moving it closer or further to the surface on which the shadow projects to, you can see that the shadow changes brightness and you may notice a slight ring of light surrounding the shadow. In essence myopia corrective lenses makes parallel rays of light divergent, so that if you shine a light through it the rays now have to cover a larger area, so the area directly under the lens is darker than the surrounding. Just outside this "shadow" you can maybe see a slight halo. This is the place where it receives light from both the divergent rays from the lenses and also directly from your light source. Hyperopia corrective lenses are the opposite, they're like magnifying glasses and will form a bright spot in the centre when you try the same thing. --antilivedT | C | G 23:13, 7 November 2009 (UTC)
- Wow, that's really bizarre that four of us used the wrong word! On a multiple choice test, I'd bet all four of us would correctly answer that lenses work by refraction, not diffraction. But somehow the wrong word stuck once it entered our heads. It's like some kind of groupthink or something. Red Act (talk) 01:32, 8 November 2009 (UTC)
- As far as I know no corrective glasses work by diffraction but rather refraction. I'm assuming the OP has myopia as my glasses do the same thing too. If you try moving it closer or further to the surface on which the shadow projects to, you can see that the shadow changes brightness and you may notice a slight ring of light surrounding the shadow. In essence myopia corrective lenses makes parallel rays of light divergent, so that if you shine a light through it the rays now have to cover a larger area, so the area directly under the lens is darker than the surrounding. Just outside this "shadow" you can maybe see a slight halo. This is the place where it receives light from both the divergent rays from the lenses and also directly from your light source. Hyperopia corrective lenses are the opposite, they're like magnifying glasses and will form a bright spot in the centre when you try the same thing. --antilivedT | C | G 23:13, 7 November 2009 (UTC)
Batteries in plastic bags
If I throw multiple AAA batteries or AA batteries in plastic bags, like Ziploc bags, will a circuit, however weak, be formed by means of the plastic bag touching the ends; and will the batteries deplete faster than if I throw them in a drawer, or loose in a plastic tub, or in a paper bag? Tempshill (talk) 19:41, 7 November 2009 (UTC)
- I'd say there would be no noticeable difference, since plastic is essentially an insulator. That is, unless the plastic is wet. StuRat (talk) 19:46, 7 November 2009 (UTC)
- A clean dry plastic bag will not complete a circuit. There is a remote possibility that a number of batteries could arrange themselves to complete a circuit. Edison (talk) 20:15, 7 November 2009 (UTC)
- Yes agreed, unless the bag is an antistatic bag used for storing electronic parts. These are usually either obviously metallic or else are slightly pink. @Edison, to form a complete loop the batteries need space enough to form something like a 3-foot circle - bigger than most plastic bags, so unlikely. In the old days the negative terminal of the battery, which is also the case, appeared as a ring around the positive terminal so it was very easy to short them out. All good quality brands now have insulated cases so this can no longer happen. SpinningSpark 21:37, 7 November 2009 (UTC)
- Nine-volt batteries are also very prone to being shorted if stored loosely, due to the proximity of the terminals. Mitch Ames (talk) 01:08, 8 November 2009 (UTC)
- Yes agreed, unless the bag is an antistatic bag used for storing electronic parts. These are usually either obviously metallic or else are slightly pink. @Edison, to form a complete loop the batteries need space enough to form something like a 3-foot circle - bigger than most plastic bags, so unlikely. In the old days the negative terminal of the battery, which is also the case, appeared as a ring around the positive terminal so it was very easy to short them out. All good quality brands now have insulated cases so this can no longer happen. SpinningSpark 21:37, 7 November 2009 (UTC)
- A clean dry plastic bag will not complete a circuit. There is a remote possibility that a number of batteries could arrange themselves to complete a circuit. Edison (talk) 20:15, 7 November 2009 (UTC)
- Many small cells have a steel cylinder around them, like Duracell, perhaps painted, which could complete a circuit unless the paint is a good insulator. A 9 volt is the most likely culprit to short in a bag of batteries, agreed, or any cells in a pocket with change and keys (not the same as the OP's scenario). Edison (talk) 04:03, 8 November 2009 (UTC)
I took these photos of animals for WP, but I'm not sure what they all are =(
If anyone knows who any of these guys are it would be greatly appreciated and would probably help various taxa get an image! Best regards, -Craig Pemberton (talk) 21:24, 7 November 2009 (UTC)
- There are 174 pictures in there. Which ones do you want identified?! It would be a good idea to post the ones you want identified as thumbnails on this page, with numbers or tags of some sort; otherwise we may not even be talking about the same picture at any given instant. Please only post the best ones. --Dr Dima (talk) 21:45, 7 November 2009 (UTC)
- Those are pretty nice, but it's clear you know what a lot of them are—you're making me kind of have to wade through them all on the off-chance I will find one of the ones you don't know and then will know what it is. You could facilitate this a little bit... (And I will disagree with Dr Dima that posting all of them is probably useful, though obviously not all will end up in articles. As a graphic designer, I have gotten a LOT of use out of high-res images on Commons that were not the "best" but were well categorized. Web space is cheap! But the blurry little beetle probably isn't worth uploading.) Your photo of the convergent evolution image should not be uploaded (the substantive content is copyrighted by the museum unless otherwise indicated—taking a new picture of a copyright image does not substantially change its copyright status). --Mr.98 (talk) 21:49, 7 November 2009 (UTC)
- Okay. Should I upload some to commons, and make a gallery here? Or should I just link the most mysterious ones? Is it alright to leave the convergent evolution one on Flickr? Thanks for the feedback! -Craig Pemberton (talk) 22:07, 7 November 2009 (UTC)
- As I said, you should only upload the best ones; definitely not all of them!!! Sorry if that was not clear. Look for similar pictures available in Wikimedia Commons, and only upload ones that are better than the ones already found in Commons. Do not upload any of the unsharp / unclear / partially obstructed ones. Also, if you have there a picture of critter not represented in Commons at all, you can upload that, as well. That should shorten your list from 174 to anything between 2 and 20. --Dr Dima (talk) 22:29, 7 November 2009 (UTC)
Rechargeable batteries
I've noticed that if the battery in my DS dies and I'm somewhere where I can't recharge it, the battery will gradually build up a charge again by itself. Why is this? --70.247.249.87 (talk) 21:51, 7 November 2009 (UTC)
Me too -- my phone will show a low battery level, then suddenly go back up. I also notice that while it's charging and the battery level is say, 70%, disconnecting the charger will make it rise to 80% -- but connecting it back again will make it drop to 70%. My hypothesis is that what is happening that less of the battery capacity becomes visible to the phone after the charger disconnecs, but this hidden capacity is later "rediscovered"? John Riemann Soong (talk) 23:21, 7 November 2009 (UTC)
- There is a phenomenon in many kinds of battery called "polarization" - it has the effect of causing the battery to lose power before it's actually totally dead. Turning the thing off and letting it recover for a few seconds allow you to get a little more use out of the battery. You can see this effect in all sorts of batteries and in all sorts of situation - so it doesn't surprise me that you're seeing it in your Nintendo DS. To be 100% clear though - the battery isn't "building up a charge" - it always had that charge - it just wouldn't let you use it. It doesn't matter how long you wait beyond the first few tens of seconds, you get to use that last little bit that was in the battery - and that's it. SteveBaker (talk) 23:22, 7 November 2009 (UTC) Added link to article. Mitch Ames (talk) 01:16, 8 November 2009 (UTC)
- So how is it a DS which I played out of battery has twice now managed to "build up" in excess of an hours' charge? I know I haven't charged it without remembering because I lost the charger. Vimescarrot (talk) 00:14, 8 November 2009 (UTC)
- For what it's worth, I've also noticed this phenomena with my DS. If I run the battery down until it shuts itself off and refuses to turn back on, and then leave it off for a while. (Ten seconds isn't enough, but ten minutes is) I can usually get at least another half hour of play out of it. Sometimes I can do this a few times before it stops working entirely. Must be a quirk of these batteries as they age. They "die" well before they're actually "empty". APL (talk) 00:50, 8 November 2009 (UTC)
- I have to leave mine for days or more to get this result. Vimescarrot (talk) 01:52, 8 November 2009 (UTC)
- For what it's worth, I've also noticed this phenomena with my DS. If I run the battery down until it shuts itself off and refuses to turn back on, and then leave it off for a while. (Ten seconds isn't enough, but ten minutes is) I can usually get at least another half hour of play out of it. Sometimes I can do this a few times before it stops working entirely. Must be a quirk of these batteries as they age. They "die" well before they're actually "empty". APL (talk) 00:50, 8 November 2009 (UTC)
- So how is it a DS which I played out of battery has twice now managed to "build up" in excess of an hours' charge? I know I haven't charged it without remembering because I lost the charger. Vimescarrot (talk) 00:14, 8 November 2009 (UTC)
Luxon theory concerning tardyons
Does Wikipedia have any information on luxon theory, specifically on their relationship to tardyons? —Preceding unsigned comment added by Active Galactic Nuclei (talk • contribs) 22:03, 7 November 2009 (UTC)
- A "luxon" is a massless particle - either a photon or a gluon. Since there are no free gluons - you might as well say skip the fancy language and say "photon" instead of "luxon". Tardyon is another neologism used (rarely) to mean anything that's moving slower than light - which is to say: Everything except photons. So you're asking whether there is information on photon theory, specifically as they relate to all normal matter? Well, yes, of course! How light and other electromagnetic radiation interacts with matter? Start with Newton - work your way forwards. SteveBaker (talk) 22:28, 7 November 2009 (UTC)
- A Google search for "luxon theory" only turned up www.tardyon.de, which I guess is what prompted this question. It's a crackpot site. However, it's right when it says that "tardyons are luxons". In field theory (classical or quantum) it's useful to describe massive waves as massless waves that interact with each other in a certain way. Specifically, a wave can be divided into "left-handed" and "right-handed" parts which individually propagate at the speed of light, and the mass (if any) shows up as an interaction between those parts. In the Standard Model that interaction also involves the Higgs field, which is why the Higgs is said to "give mass" to the particles. -- BenRG (talk) 23:14, 7 November 2009 (UTC)
Here was my starting point: http://en.wikipedia.org/wiki/Massless_particle. I was hoping for anything more you might have on it? —Preceding unsigned comment added by Active Galactic Nuclei (talk • contribs) 23:46, 7 November 2009 (UTC)
why isn't the Wolf-Kishner reduction or the Clemmensen reduction used for meth synthesis?
Bleach (sodium hypochlorite -- failing that one could fall back to Swern oxidation) and W-K or Clemmensen would seem to do the job quite nicely. No phosphine gas? Or is red phosphorus much cheaper? (This is purely out of curiosity as I reduce an alcohol to a C-H bond for the umpteenth time on paper...) John Riemann Soong (talk) 23:39, 7 November 2009 (UTC)
- Bleach has its own problems, see Bleach#Chemical interactions. My guess is that most people home-brewing their own crystal meth aren't exactly familiar with various reaction pathways that get them their product. Likely one could do it safer with the right materials. But really, these guys are just following a recipe without knowing why they do each step, and what all the process is about. --Jayron32 01:24, 8 November 2009 (UTC)
- Well, couldn't the amine group be protected under acidic conditions? Or is the N-H bond that reacts? Plus even if one gets a chlorinated amine, wouldn't the chlorines be relatively easy to take off in the presence of acid, seeing how chloride a good leaving group? John Riemann Soong (talk) 01:49, 8 November 2009 (UTC)
- Again, you're asking why people don't follow good organic synthesis procedures when they are brewing crystal meth in their garage. The answer is because they aren't chemists; they're gap-toothed hillbillies who didn't finish the 4th grade and are following a recipe they found on the internet. People engaged in illegal activities don't often have the time and resources to do proper, standardized experiments to work out alternate pathways. They have a recipe, they follow it, they blow up their garage when they screw up. Questioning why they don't "do it right" misses the point. It was much the same with white lightning compared to legal, regulated booze. --Jayron32 02:00, 8 November 2009 (UTC)
- Well 2 questions. Does bleach oxidise alcohols under acidic conditions? How do you lyse a quartenary ammonium salt? I mean, street drug syntheses often have a fair amount of pragmatics built into them -- clearly there are clandestine chemists who cooperate with gangs or new synthesis techniques wouldn't pop up now and then. John Riemann Soong (talk) 02:06, 8 November 2009 (UTC)
- Again, you're asking why people don't follow good organic synthesis procedures when they are brewing crystal meth in their garage. The answer is because they aren't chemists; they're gap-toothed hillbillies who didn't finish the 4th grade and are following a recipe they found on the internet. People engaged in illegal activities don't often have the time and resources to do proper, standardized experiments to work out alternate pathways. They have a recipe, they follow it, they blow up their garage when they screw up. Questioning why they don't "do it right" misses the point. It was much the same with white lightning compared to legal, regulated booze. --Jayron32 02:00, 8 November 2009 (UTC)
energy vs frequency
The articles on frequency and energy confirm the increased frequency requires or represents increased energy but where can I find a plot of energy versus frequency for both sound and electromagnetic waves and all types of vibrations from zero frequency to Gamma and Cosmic rays? 71.100.0.254 (talk) 23:46, 7 November 2009 (UTC)
- For light the formula is: E=hf, where E is energy per photon, h is Planck's constant and f is frequency. That holds for any EM radiation. Cosmic rays are just particles, they aren't usually thought of as waves (although you can get a frequency from the de Broglie wavelength if you want to, in which case the same formula will hold as for light). For sound, you can't divide it into particles, so there isn't a discrete energy. There is sound energy flux (not a great article, though...), which is the amount of energy to pass through a unit area in a unit time. --Tango (talk) 00:25, 8 November 2009 (UTC)
- Actually, sound felt left out of the whole "wave-particle duality" party, so it went and got itself its own sound particle. Well, sort of. But there is a particle equivalent for vibrational energy just like there is one for electromagnetic energy. --Jayron32 01:19, 8 November 2009 (UTC)
- Big "sort of"! As the first line of that article says, it only applies is a solid crystal lattice. We usually think of sound as being carried by air, since that is what we can hear, and air isn't a solid crystal lattice. --Tango (talk) 02:05, 8 November 2009 (UTC)
- But what about a graph... x = frequency, y = energy? 71.100.0.254 (talk) —Preceding undated comment added 01:47, 8 November 2009 (UTC).
- They're proportional, so it's a straight line, and h is positive, so it goes from bottom left to top right. --Tango (talk) 02:05, 8 November 2009 (UTC)
- So then hf=mc2...? 71.100.0.254 (talk) 02:39, 8 November 2009 (UTC)
- Kind of. That only works if you take m as relativistic mass. The rest mass of a photon is zero. --Tango (talk) 02:43, 8 November 2009 (UTC)
- In my mind it appears I was actually thinking of a curve so I must have been thing of wavelength and not frequency. Anyway the would mean that since wavelength is inversely proportional to frequency that there must be a limit on the shortness of a wavelength (the shortest possible wavelength) to equal the greatest amount of relativistic energy for every particle? 71.100.0.254 (talk) 02:47, 8 November 2009 (UTC)
- Mathematically, there is no limit to how small a wavelength can be, or therefore how much energy a photon can have. However, any wavelength shorter than a Planck length is likely to be meaningless, so the universe appears to have a practical limit built in. --Jayron32 02:53, 8 November 2009 (UTC)
- In my mind it appears I was actually thinking of a curve so I must have been thing of wavelength and not frequency. Anyway the would mean that since wavelength is inversely proportional to frequency that there must be a limit on the shortness of a wavelength (the shortest possible wavelength) to equal the greatest amount of relativistic energy for every particle? 71.100.0.254 (talk) 02:47, 8 November 2009 (UTC)
- Kind of. That only works if you take m as relativistic mass. The rest mass of a photon is zero. --Tango (talk) 02:43, 8 November 2009 (UTC)
- So then hf=mc2...? 71.100.0.254 (talk) 02:39, 8 November 2009 (UTC)
- They're proportional, so it's a straight line, and h is positive, so it goes from bottom left to top right. --Tango (talk) 02:05, 8 November 2009 (UTC)
- Actually, sound felt left out of the whole "wave-particle duality" party, so it went and got itself its own sound particle. Well, sort of. But there is a particle equivalent for vibrational energy just like there is one for electromagnetic energy. --Jayron32 01:19, 8 November 2009 (UTC)
Somehow I'm thinking that at that wavelength something mysterious happens like the propagation in a single direction stops and you get a stationary oscillation that is self containing otherwise known as energy in the form of mass. Sort of like a cowboy rope trick where the rope spins in a loop and then the loop moves forward and backward or some such crazy back and forth but stationary relationship like that. 71.100.0.254 (talk) 03:15, 8 November 2009 (UTC)
November 8
Convict Fish
In the BBC Life natural world documentary about fish there is a segment about a fish called the "convict fish", this is the clip [35] and I was trying to find out more about it and the like, however searches only ield the convict chichlids, which seem quite different and no where near as peculiar. Any idea what its taxonomical name is / any info on it? MedicRoo (talk) 01:09, 8 November 2009 (UTC)
- See this page. We have an article at Pholidichthys leucotaenia, but it's not very informative. Deor (talk) 01:57, 8 November 2009 (UTC)
Nimh substitute for Nicad?
Can an Nimh battery of the same voltage and millampere-hour rating be used to replace a Nicad battery in a cordless phone? I thought not, because the charging arrangements can be different. Edison (talk) 03:59, 8 November 2009 (UTC)
Kinematics Question
A rock is dropped from a seacliff and the sound of it striking the ocean is heard 3.0 s later. If the speed of sound is 340 m/s, how high is the cliff? The answer is 41 meters, but I don't know how to get it. —Preceding unsigned comment added by 174.6.144.211 (talk) 04:21, 8 November 2009 (UTC)
- Call the height of the cliff h. Then work out how long it will take to fall that far (remember your constant acceleration formulae?) and how long it will take for the sound to travel that far. Then add them together and set it equal to 3 and solve for h. --Tango (talk) 04:35, 8 November 2009 (UTC)
- The question is ambiguous and poorly framed because it does not specify where the listener is. The sound might well be heard by someone at the water level below the cliff, or 100 meters from the cliff in a boat, just as it might be heard by someone on the cliff. Edison (talk) 04:53, 8 November 2009 (UTC)
- The question only makes sense if they are listening from the top of the cliff. If they are at the water level below the cliff it would be a trick question, since the sound thing wouldn't add anything. If they are in a boat elsewhere on the water then there isn't enough information in the question to answer it. While it would be good if the question were more precise we shouldn't over analyse things - if it is obvious what the question means then it is much better to answer it rather than pick holes in it. --Tango (talk) 05:15, 8 November 2009 (UTC)
- (ec) The speed of sound is a red herring. Solve the problem assuming the speed of sound is instantaneous and see how it works out. Matt Deres (talk) 04:56, 8 November 2009 (UTC)
- I assume the problem is that the correction for the error caused by the speed of sound is kind of nonlinear. If you simply subtract away the error caused and add it back to the time (say 3.12 seconds) that changes your answer and now you have to iteratively calculate your solution. John Riemann Soong (talk) 05:01, 8 November 2009 (UTC)
- That's not true. The speed of sound will make a difference. If the speed of sound were instantaneous you would get a larger value for the height of the cliff. --Tango (talk) 05:15, 8 November 2009 (UTC)
- The question is ambiguous and poorly framed because it does not specify where the listener is. The sound might well be heard by someone at the water level below the cliff, or 100 meters from the cliff in a boat, just as it might be heard by someone on the cliff. Edison (talk) 04:53, 8 November 2009 (UTC)
are quantised values always rational?
The cardinality of the continuum provides much more than the cardinality of the natural numbers... if the wavelength of a photon is 1500/pi nm, and a HOMO-LUMO gap of a fluorescent molecule is 1500/(pi+1/e^99) nm, will excitation occur? John Riemann Soong (talk) 05:06, 8 November 2009 (UTC)
- I think the difference in length there (I haven't actually calculated it) will be less than the Planck length, which means they can be assumed to be equal. Quoting lengths at greater precision than +/- the Planck length is pretty meaningless. --Tango (talk) 05:19, 8 November 2009 (UTC)
- That said, the largest difference that still allows for excitation is probably larger than the Planck length, otherwise it would hardly ever occur. I know virtually nothing about HOMO-LUMO gaps, though, so I'll have to let someone else give a more specific answer to that problem. In general though, nothing is exact in physics, so we can always choose to use a countable subset of the real numbers (eg. truncate all decimals after a few hundred places, it won't make any difference to anything - a few dozen places would probably suffice for almost everything). --Tango (talk) 05:23, 8 November 2009 (UTC)
































