Antimony: Difference between revisions
m Reverted edits by 71.60.181.223 (talk) to last version by Tide rolls |
No edit summary |
||
| Line 23: | Line 23: | ||
*used in [[type metal]], e.g. for [[Linotype machine|linotype]] printing machines |
*used in [[type metal]], e.g. for [[Linotype machine|linotype]] printing machines |
||
*used in [[pewter]] |
*used in [[pewter]] |
||
*used to harden alloys with low tin content in the manufacturing of organ pipes |
|||
Antimony compounds in the form of [[oxide]]s, [[sulfide]]s, sodium antimonate, and antimony trichloride are used in the making of flame-proofing compounds, [[ceramic]] enamels, [[glass]], [[paint]]s, and [[pottery]]. [[Antimony trioxide]] is the most important of the antimony compounds and is primarily used in flame-retardant formulations. These flame-retardant applications include such markets as children's clothing, toys, aircraft and automobile seat covers. It is also used in the fiberglass composites industry as an additive to polyester resins for such items as light aircraft engine covers. The resin will burn while a flame is held to it but will extinguish itself as soon as the flame is removed. Antimony sulfide is also one of the ingredients of [[Match#Safety matches|safety matches]]. |
Antimony compounds in the form of [[oxide]]s, [[sulfide]]s, sodium antimonate, and antimony trichloride are used in the making of flame-proofing compounds, [[ceramic]] enamels, [[glass]], [[paint]]s, and [[pottery]]. [[Antimony trioxide]] is the most important of the antimony compounds and is primarily used in flame-retardant formulations. These flame-retardant applications include such markets as children's clothing, toys, aircraft and automobile seat covers. It is also used in the fiberglass composites industry as an additive to polyester resins for such items as light aircraft engine covers. The resin will burn while a flame is held to it but will extinguish itself as soon as the flame is removed. Antimony sulfide is also one of the ingredients of [[Match#Safety matches|safety matches]]. |
||
Revision as of 20:28, 9 November 2009
 | ||||||||||||||||||||||||||
| Antimony | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | ||||||||||||||||||||||||||
| Appearance | silvery lustrous gray | |||||||||||||||||||||||||
| Standard atomic weight Ar°(Sb) | ||||||||||||||||||||||||||
| Antimony in the periodic table | ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| Atomic number (Z) | 51 | |||||||||||||||||||||||||
| Group | group 15 (pnictogens) | |||||||||||||||||||||||||
| Period | period 5 | |||||||||||||||||||||||||
| Block | p-block | |||||||||||||||||||||||||
| Electron configuration | [Kr] 4d10 5s2 5p3 | |||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 18, 5 | |||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||
| Melting point | 903.78 K (630.63 °C, 1167.13 °F) | |||||||||||||||||||||||||
| Boiling point | 1908 K (1635 °C, 2975 °F) | |||||||||||||||||||||||||
| Density (at 20° C) | 6.694 g/cm3[3] | |||||||||||||||||||||||||
| when liquid (at m.p.) | 6.53 g/cm3 | |||||||||||||||||||||||||
| Heat of fusion | 19.79 kJ/mol | |||||||||||||||||||||||||
| Heat of vaporization | 193.43 kJ/mol | |||||||||||||||||||||||||
| Molar heat capacity | 25.23 J/(mol·K) | |||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||
| Oxidation states | common: −3, +3, +5 −2,[4] −1,[4] 0,[5] +1,[6] +2,? +4[7] | |||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.05 | |||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||
| Atomic radius | empirical: 140 pm | |||||||||||||||||||||||||
| Covalent radius | 139±5 pm | |||||||||||||||||||||||||
| Van der Waals radius | 206 pm | |||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||
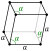
| Crystal structure | rhombohedral (hR2) | |||||||||||||||||||||||||
| Lattice constants | a = 0.45066 nm α = 57.112° ah = 0.43084 nm ch = 1.12736 nm (at 20 °C)[3] | |||||||||||||||||||||||||
| Thermal expansion | 11.04×10−6/K (at 20 °C)[a] | |||||||||||||||||||||||||
| Thermal conductivity | 24.4 W/(m⋅K) | |||||||||||||||||||||||||
| Electrical resistivity | 417 nΩ⋅m (at 20 °C) | |||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[8] | |||||||||||||||||||||||||
| Molar magnetic susceptibility | −99.0×10−6 cm3/mol[9] | |||||||||||||||||||||||||
| Young's modulus | 55 GPa | |||||||||||||||||||||||||
| Shear modulus | 20 GPa | |||||||||||||||||||||||||
| Bulk modulus | 42 GPa | |||||||||||||||||||||||||
| Speed of sound thin rod | 3420 m/s (at 20 °C) | |||||||||||||||||||||||||
| Mohs hardness | 3.0 | |||||||||||||||||||||||||
| Brinell hardness | 294–384 MPa | |||||||||||||||||||||||||
| CAS Number | 7440-36-0 | |||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||
| Discovery | Arabic alchemists (before AD 815) | |||||||||||||||||||||||||
| Symbol | "Sb": from Latin stibium 'stibnite' | |||||||||||||||||||||||||
| Isotopes of antimony | ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
Antimony (Template:Pron-en AN-ti-mo-nee)[note 1] is a chemical element with the symbol Sb (Template:Lang-la, meaning "mark") and atomic number 51. A metalloid, antimony has four allotropic forms. The stable form of antimony is a blue-white metalloid. Yellow and black antimony are unstable non-metals. Antimony is used in electronics and flame-proofing, in paints, rubber, ceramics, enamels, drugs to treat Leishmania infection and a wide variety of alloys.
Properties
Antimony in its elemental form is a silvery white, brittle, fusible, crystalline solid that exhibits poor electrical and heat conductivity properties and vaporizes at low temperatures. A metalloid, antimony resembles a metal in its appearance and in many of its physical properties, but does not chemically react as a metal. It is reactive with oxidizing acids and halogens. Antimony and some of its alloys are unusual in that they expand on cooling. Antimony is geochemically categorized as a chalcophile, occurring with sulfur and the heavy metals lead, copper, and silver.
The abundance of antimony in the Earth's crust is estimated at 0.2 to 0.5 parts per million.[11]
Applications
Antimony is increasingly being used in the semiconductor industry in the production of diodes, infrared detectors, and Hall-effect devices. As an alloy, this metalloid greatly increases lead's hardness and mechanical strength. The most important use of antimony is as a hardener in lead for storage batteries. Uses include:
- batteries
- antifriction alloys, such as Babbit metal
- small arms, buckshot, and tracer ammunition
- cable sheathing
- matches
- medications such as antiprotozoan drugs
- HF pH electrode construction
- plumbing
- soldering - some "lead-free" solders contain 5% Sb
- used in the past to treat Schistosomiasis; today Praziquantel is universally used
- used in type metal, e.g. for linotype printing machines
- used in pewter
- used to harden alloys with low tin content in the manufacturing of organ pipes
Antimony compounds in the form of oxides, sulfides, sodium antimonate, and antimony trichloride are used in the making of flame-proofing compounds, ceramic enamels, glass, paints, and pottery. Antimony trioxide is the most important of the antimony compounds and is primarily used in flame-retardant formulations. These flame-retardant applications include such markets as children's clothing, toys, aircraft and automobile seat covers. It is also used in the fiberglass composites industry as an additive to polyester resins for such items as light aircraft engine covers. The resin will burn while a flame is held to it but will extinguish itself as soon as the flame is removed. Antimony sulfide is also one of the ingredients of safety matches.
In the 1950s, tiny beads of a lead-antimony alloy were used for the emitters and collectors of NPN alloy junction transistors. [citation needed]
The natural sulfide of antimony, stibnite, was known and used in Biblical times, as a medication and in Islamic/Pre-Islamic times as a cosmetic. The Sunan Abi Dawood reports, “Muhammad said: 'Among the best types of collyrium is antimony (ithmid) for it clears the vision and makes the hair sprout.'”[12]
Stibnite is still used in some developing countries as a medication. Antimony has been used for the treatment of schistosomiasis. Antimony attaches itself to sulfur atoms in certain enzymes which are used by both the parasite and human host. Small doses can kill the parasite without causing damage to the patient. Antimony and its compounds are used in several veterinary preparations like Anthiomaline or Lithium antimony thiomalate, which is used as a skin conditioner in ruminants. Antimony has a nourishing or conditioning effect on keratinized tissues, at least in animals. Tartar emetic is another antimony preparation which is used as an anti-schistosomal drug. Treatments chiefly involving antimony have been called antimonials.
Antimony-based drugs such as allopurinol and Meglumine, are also considered the drugs of choice for the treatment of leishmaniasis in domestic animals. Unfortunately, as well as having low therapeutic indices, the drugs are poor at penetrating the bone marrow, where some of the Leishmania amastigotes reside, and so cure of the disease - especially the visceral form - is very difficult.
A coin made of antimony was issued in the Keichow Province of China in 1931. The coins were not popular, being too soft and they wore quickly when in circulation. After the first issue no others were produced.[13]
Etymology
The ancient words for antimony mostly have, as their chief meaning, kohl, the sulfide of antimony. Pliny the Elder, however, distinguishes between male and female forms of antimony; his male form is probably the sulfide, the female form, which is superior, heavier, and less friable, is probably native metallic antimony.[14]
The Egyptians called antimony mśdmt; in hieroglyphics, the vowels are uncertain, but there is an Arabic tradition that the word is mesdemet.[15] The Greek word, stimmi, is probably a loan word from Arabic or Egyptian, and is used by the Attic tragic poets of the 5th century BC; later Greeks also used stibi, as did Celsus and Pliny, writing in Latin, in the first century AD. Pliny also gives the names stimi [sic], larbaris, alabaster, and the "very common" platyophthalmos, "wide-eye" (from the effect of the cosmetic). Later Latin authors adapted the word to Latin as stibium. The Arabic word for the substance, as opposed to the cosmetic, can appear as ithmid, athmoud, othmod, or uthmod. Littré suggests the first form, which is the earliest, derives from stimmida, (one) accusative for stimmi.[16]
The use of Sb as the standard chemical symbol for antimony is due to the 18th century chemical pioneer, Jöns Jakob Berzelius, who used this abbreviation of the name stibium.

The medieval Latin form, from which the modern languages and late Byzantine Greek, take their names, is antimonium. The origin of this is uncertain; all suggestions have some difficulty either of form or interpretation. The popular etymology, from anti-monachos or French antimoine, still has adherents; this would mean "monk-killer", and is explained by many early alchemists being monks, and antimony being poisonous.[note 2] So does the hypothetical Greek word antimonos, "against one", explained as "not found as metal", or "not found unalloyed".[17] Lippmann conjectured a Greek word, anthemonion, which would mean "floret", and he cites several examples of related Greek words (but not that one) which describe chemical or biological efflorescence.[18]
The early uses of antimonium include the translations, in 1050-1100, by Constantine the African of Arabic medical treatises.[19] Several authorities believe that antimonium is a scribal corruption of some Arabic form; Meyerhof derives it from ithmid;[20] other possibilities include Athimar, the Arabic name of the metal, and a hypothetical *as-stimmi, derived from or parallel to the Greek.[21]
History

Antimony's sulfide compound, antimony (III) trisulfide, Sb2S3 was recognized in antiquity, at least as early as 3000 BC. Pastes of Sb2S3 powder in fat[22] or in other materials have been used since that date as eye cosmetics in the Middle East and farther afield; in this use, Sb2S3 is called kohl. It was used to darken the brows and lashes, or to draw a line around the perimeter of the eye.
An artifact made of antimony dating to about 3000 BC was found at Tello, Chaldea (part of present-day Iraq), and a copper object plated with antimony dating between 2500 BC and 2200 BC has been found in Egypt.[23] There is some uncertainty as to the description of the artifact from Tello. Although it is sometimes reported to be a vase, a recent detailed discussion of reports it to be rather a fragment of indeterminate purpose.[24] The first European description of a procedure for isolating antimony is in the book De la pirotechnia of 1540 by Vannoccio Biringuccio, written in Italian. This book precedes the more famous 1556 book in Latin by Agricola, De re metallica, even though Agricola has been often incorrectly credited with the discovery of metallic antimony. A text describing the preparation of metallic antimony that was published in Germany in 1604 purported to date from the early fifteenth century, and if authentic it would predate Biringuccio. The book, in German, was the Triumph Wagen Antimonii (Triumphal Chariot of Antimony), and its putative author was a certain Benedictine monk, writing under the name Basilius Valentinus. Already in 1710 Wilhelm Gottlob Freiherr von Leibniz, after careful inquiry, concluded that the work was spurious, that there was no monk named Basilius Valentinus, and the book's author was its ostensible editor, Johann Thölde (ca. 1565-ca. 1624). There is now agreement among professional historians that the Triumph Wagen was written after the middle of the sixteenth century and that Thölde was likely its author.[25] An English translation of the Triumph Wagen appeared in English in 1660, under the title The Triumphant Chariot of Antimony. The work remains of great interest, chiefly because it documents how followers of the renegade German physician, Philippus Theophrastus Paracelsus von Hohenheim (of whom Thölde was one), came to associate the practice of alchemy with the preparation of chemical medicines.
According to the traditional history of Middle Eastern alchemy, pure antimony was well known to Geber, sometimes called "the Father of Chemistry", in the 8th century. Here there is still an open controversy: Marcellin Berthelot, who translated a number of Geber's books, stated that antimony is never mentioned in them, but other authors[who?] claim that Berthelot translated only some of the less important books, while the more interesting ones (some of which might describe antimony) are not yet translated, and their content is completely unknown.
The first natural occurrence of pure antimony ('native antimony') in the Earth's crust was described by the Swedish scientist and local mine district engineer Anton von Swab in 1783. The type-sample was collected from the Sala Silver Mine in the Bergslagen mining district of south central Sweden.
Production


Even though this element is not abundant, it is found in over 100 mineral species. Antimony is sometimes found native, but more frequently it is found in the sulfide stibnite (Sb2S3) which is the predominant ore mineral. Commercial forms of antimony are generally ingots, broken pieces, granules, and cast cake. Other forms are powder, shot, and single crystals.
In 2005, China was the top producer of antimony with about 84% world share followed at a distance by South Africa, Bolivia and Tajikistan, reports the British Geological Survey.
| Country | Tonnes | % of total |
|---|---|---|
| 126,000 | 84.0 | |
| 6,000 | 4.0 | |
| 5,225 | 3.5 | |
| 4,073 | 2.7 | |
| 3,000 | 2.0 | |
| Top 5 | 144,298 | 96.2 |
| Total world | 150,000 | 100.0 |
Chiffres de 2003, métal contenue dans les minerais et concentrés, source: L'état du monde 2005 Template:Fr
The largest mine in China is Xikuangshan mine in Hunan Province.
Precautions
Antimony and many of its compounds are toxic. Clinically, antimony poisoning is very similar to arsenic poisoning. In small doses, antimony causes headache, dizziness, and depression. Larger doses cause violent and frequent vomiting, and will lead to death in a few days.
Antimony leaches from polyethylene terephthalate (PET) bottles into bottled water, but at levels below drinking water guidelines.[26][27] The guidelines are:
- World Health Organization: 20 µg/L
- Japan: 15 µg/L[28]
- United States Environmental Protection Agency, Health Canada and the Ontario Ministry of Environment: 6 µg/L
- German Federal Ministry of Environment: 5 µg/L[29]
The acidic nature of the drink is sufficient to dissolve small amounts of antimony trioxide contained in the packaging of the drink;[citation needed] modern manufacturing methods prevent this occurrence.[citation needed] The longer the beverage has been bottled and the higher the temperature, the more antimony is leached.[30]
Compounds
Important compounds of antimony include:
- Antimony pentafluoride SbF5
- Antimony trioxide Sb2O3
- Stibine (antimony trihydride SbH3)
- Indium antimonide (InSb)
- Fluoroantimonic acid (HSbF6)
See also
Notes
References
- ^ "Standard Atomic Weights: Antimony". CIAAW. 1993.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ a b Sb(−2) and Sb(−1) has been observed in [Sb2]4− and 1∞[Sbn]n−, respectively; see Boss, Michael; Petri, Denis; Pickhard, Frank; Zönnchen, Peter; Röhr, Caroline (2005). "Neue Barium-Antimonid-Oxide mit den Zintl-Ionen [Sb]3−, [Sb2]4− und 1∞[Sbn]n− / New Barium Antimonide Oxides containing Zintl Ions [Sb]3−, [Sb2]4− and 1∞[Sbn]n−". Zeitschrift für Anorganische und Allgemeine Chemie (in German). 631 (6–7): 1181–1190. doi:10.1002/zaac.200400546.
- ^ Anastas Sidiropoulos (2019). "Studies of N-heterocyclic Carbene (NHC) Complexes of the Main Group Elements" (PDF). p. 39. doi:10.4225/03/5B0F4BDF98F60. S2CID 132399530.
- ^ Sb(I) have been observed in organoantimony compounds; see Šimon, Petr; de Proft, Frank; Jambor, Roman; Růžička, Aleš; Dostál, Libor (2010). "Monomeric Organoantimony(I) and Organobismuth(I) Compounds Stabilized by an NCN Chelating Ligand: Syntheses and Structures". Angewandte Chemie International Edition. 49 (32): 5468–5471. doi:10.1002/anie.201002209. PMID 20602393.
- ^ Sb(IV) has been observed in [SbCl6]2−, see Nobuyoshi Shinohara; Masaaki Ohsima (2000). "Production of Sb(IV) Chloro Complex by Flash Photolysis of the Corresponding Sb(III) and Sb(V) Complexes in CH3CN and CHCl3". Bulletin of the Chemical Society of Japan. 73 (7): 1599–1604. doi:10.1246/bcsj.73.1599.
- ^ Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds". CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ "Antimony Statistics and Information". United States Geological Survey. 2009-01-31. Retrieved 2009-04-15.
- ^ Sunan Abu-Dawud (Ahmad Hasan translation). Book 32, Number 4050.
- ^ "Metals Used in Coins and Medals". ukcoinpics.co.uk. Retrieved 2009-10-16.
- ^ Pliny, Natural history, 33.33; W.H.S. Jones, the Loeb Classical Library translator, supplies a note suggesting the identifications.
- ^ Albright, p.230; Sarton p.541, quotes Meyerhof, the translator of the book he is reviewing.
- ^ LSJ, s.v., vocalisation, spelling, and declension vary; Endlich, p.28; Celsus, 6.6.6 ff; Pliny Natural History 33.33; Lewis and Short: Latin Dictionary. OED, s. "antimony".
- ^ See, for example, Diana Fernando, Alchemy : an illustrated A to Z (1998) and Kirk-Othmer (below) respectively. Fernando even derives it from the story of how "Basil Valentine" and his fellow monastic alchemists poisoned themselves by working with antimony; antimonium is found two centuries before his time. "Popular etymology" from OED; as for antimonos, the pure negative would be more naturally expressed by a- "not".
- ^ Lippman, p.643-5
- ^ Lippman, p.642, writing in 1919, says "zuerst".
- ^ Meyerhof as quoted in Sarton, p.541, asserts that ithmid or athmoud became corrupted in the medieval "traductions barbaro-latines".; the OED asserts that some Arabic form is the origin, and if ithmid is the root, posits athimodium, atimodium, atimonium, as intermediate forms.
- ^ Endlich, p.28; one of the advantages of as-stimmi would be that it has a whole syllable in common with antimonium.
- ^ Priesner and Figala
- ^ Kirk-Othmer, entry "Antimony"
- ^ The fragment was presented in a lecture in 1892. One contemporary commented, "we only know of antimony at the present day as a highly brittle and crystalline metal, which could hardly be fashioned into a useful vase, and therefore this remarkable 'find' must represent the lost art of rendering antimony malleable." Moorey 1994:241
- ^ E.g., Claus Priesner and Karin Figala, eds. (1998), Alchemie: Lexikon einer hermetischen Wissenschaft (Munich: Beck), s.v. "Basilius Valentinus." Harold Jantz was perhaps the only modern scholar to deny Thölde's authorship, but he too agrees that the work dates from after 1550: see his catalogue of German Baroque literature, available online at [1].
- ^ Shotyk, W; Krachler, M; Chen, B (2006). "Contamination of Canadian and European bottled waters with antimony from PET containers". Journal of environmental monitoring : JEM. 8 (2): 288–92. doi:10.1039/b517844b. ISSN 1464-0325. PMID 16470261.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "London Free Press:". Lfpress.com. Retrieved 2008-09-12.
- ^ H. Wakayama, Table 2, p. 84
- ^ Shotyk et al., 2006
- ^ Westerhoff, P (2008). "Antimony leaching from polyethylene terephthalate (PET) plastic used for bottled drinking water". Water Research. 42 (3): 551–556. doi:10.1016/j.watres.2007.07.048. PMID 17707454.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)
Bibliography
- W. F. Albright "Notes on Egypto-Semitic Etymology. II", The American Journal of Semitic Languages and Literatures, Vol. 34, No. 4. (Jul., 1918), pp. 215–255 (p.230)
- Endlich, F.M. "On Some Interesting Derivations of Mineral Names", The American Naturalist, Vol. 22, No. 253. (Jan., 1888), pp. 21–32 (p.28)
- Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed. 2004. Entry for antimony.
- Edmund Oscar von Lippmann (1919) Entstehung und Ausbreitung der Alchemie, teil 1. Berlin: Julius Springer. In German.
- Moorey, PRS. (1994) Ancient Mesopotamian Materials and Industries: the Archaeological Evidence. New York: Clarendon Press.
- Priesner, Claus and Figala, Karin, eds. (1998) Alchemie. Lexikon einer hermetischen Wissenschaft. München: C.H. Beck. 412 p. In German.
- Sarton, George. (1935) Review of Al-morchid fi'l-kohhl, ou Le guide d'oculistique, translated by Max Meyerhof. Isis (1935), 22(2):539-542 In French.
- Shotyk, W; Krachler, M; Chen, B (2006). "Contamination of Canadian and European bottled waters with antimony from PET containers". Journal of environmental monitoring : JEM. 8 (2): 288–92. doi:10.1039/b517844b. ISSN 1464-0325. PMID 16470261.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Public Health Statement for Antimony
- Wakayama, Hiroshi, "Revision of Drinking Water Standards in Japan", Ministry of Health, Labor and Welfare (Japan), 2003
External links
- National Pollutant Inventory - Antimony and compounds
- WebElements.com - Antimony
- Chemistry in its element podcast (MP3) from the Royal Society of Chemistry's Chemistry World: Antimony
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).