Superior mesenteric artery syndrome: Difference between revisions
m robot Adding: ja:上腸間膜動脈症候群 |
Redviolin87 (talk | contribs) |
||
| Line 56: | Line 56: | ||
== Symptoms == |
== Symptoms == |
||
Symptoms include early satiety, [[nausea]], bilious [[vomiting]] of large quantities of partially undigested food, extreme "stabbing" postprandial [[abdominal pain]] due to the duodenal compression and the compensatory reversed [[peristalsis]], abdominal distention/distortion, [[eructation]], external hypersensitivity or tenderness of the abdominal area, and severe [[malnutrition]] accompanying spontaneous [[wasting]]. <ref name="Baltazar">{{cite journal |
Symptoms include early satiety, [[nausea]], bilious [[vomiting]] of large quantities of partially undigested food, extreme "stabbing" postprandial [[abdominal pain]] (due to both the duodenal compression and the compensatory reversed [[peristalsis]]), abdominal distention/distortion, [[eructation]], external hypersensitivity or tenderness of the abdominal area, and severe [[malnutrition]] accompanying spontaneous [[wasting]]. <ref name="Baltazar">{{cite journal |
||
|author=Baltazar U, Dunn J, Floresguerra C, Schmidt L, Browder W |
|author=Baltazar U, Dunn J, Floresguerra C, Schmidt L, Browder W |
||
|title=Superior mesenteric artery syndrome: an uncommon cause of intestinal obstruction |
|title=Superior mesenteric artery syndrome: an uncommon cause of intestinal obstruction |
||
Revision as of 11:44, 2 January 2010
| Superior mesenteric artery syndrome | |
|---|---|
| Specialty | Gastroenterology |
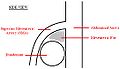
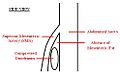
Superior mesenteric artery (SMA) syndrome is a very rare, life-threatening gastro-vascular disorder characterized by a compression of the third portion of the duodenum by the abdominal aorta (AA) and the overlying superior mesenteric artery. The syndrome is typically caused by an angle of 6°-25° between the AA and the SMA, in comparison to the normal range of 38°-56°, due to a lack of retroperitoneal fat. In addition, the aortomesenteric distance is 2-8 millimeters, as opposed to the typical 10-20. [1]
SMA syndrome was first described in 1861 by Carl Freiherr von Rokitansky in victims at autopsy, but remained pathologically undefined until 1927 when Wilkie published the first comprehensive series of 75 patients.[2] Only 0.013 - 0.3% of upper-gastrointestinal-tract barium studies support a diagnosis, making it one of the rarest gastrointestinal disorders known to medical science.[1] With only about 500 reported cases in English-language medical literature, recognition of SMA syndrome as a distinct clinical entity is controversial,[3] with some in the medical community doubting its existence entirely.[1] SMA syndrome is estimated to have a mortality rate of 1 in 3.[4]
SMA syndrome is also known as Wilkie's syndrome, cast syndrome, mesenteric root syndrome, chronic duodenal ileus and intermittent arterio-mesenteric occlusion.[5] It is distinct from Nutcracker syndrome, which is the entrapment of the left renal vein between the AA and the SMA.
Symptoms
Symptoms include early satiety, nausea, bilious vomiting of large quantities of partially undigested food, extreme "stabbing" postprandial abdominal pain (due to both the duodenal compression and the compensatory reversed peristalsis), abdominal distention/distortion, eructation, external hypersensitivity or tenderness of the abdominal area, and severe malnutrition accompanying spontaneous wasting. [6] This, in turn, increases the duodenal compression, spurring a vicious cycle.[7] Symptoms are partially relieved when in the left lateral decubitus or knee-to-chest position. A Hayes maneuver (pressure applied below the umbilicus in cephalad and dorsal direction) elevates the root of the SMA, also slightly easing the constriction. Symptoms are often aggravated when leaning to the right or taking a supine (face up) position.[6]
Causes
Under normal circumstances, retroperitoneal fat and lymphatic tissue serve as a cushion for the duodenum, protecting it from compression by the SMA. SMA syndrome is thus triggered by any condition involving an insubstantial cushion and narrow mesenteric angle. Patients predominantly have a lengthy or even lifelong history of chronic abdominal complaints, with intermittent exacerbations depending on the degree of duodenal compression. Risk factors include anatomic characteristics such as: aesthenic (very thin or "lanky") body build, an unusually high insertion of the duodenum at the ligament of Treitz, a particularly low origin of the SMA, or intestinal malrotation around an axis formed by the SMA.[8] Predisposition is easily aggravated by any of the following: poor motility of the digestive tract[5], retroperitional tumors, loss of appetite, malabsorption, cachexia, exaggerated lumbar lordosis, visceroptosis, abdominal wall laxity, peritoneal adhesions, rapid linear adolescent growth spurt, weight loss, starvation, catabolic states (as with cancer and burns), prolonged bed rest, application of body casts, left nephrectomy, spinal cord injury, or scoliosis surgery.[1] Vomiting following scoliosis surgery should be investigated thoroughly to avoid significant morbidity, protracted hospital stay and potential mortality. [9]
Demographics
SMA syndrome is extremely rare, evident in only 0.013 - 0.3% of upper-gastrointestinal-tract barium studies.[1] However, unfamiliarity with this condition in the medical community coupled with its intermittent and nonspecific symptomatology probably results in its underdiagnosis.[10]
As the syndrome involves a lack of retroperitional (essential) fat, four of every five afflicted are underweight, often to the point of sickliness and emaciation. Females are impacted twice as often as males, with 75% of cases occurring between the ages of 10 and 30. Common co-morbid conditions include hyperchlorhydria (noted in 50% of cases), peptic ulcer disease (25-45%), pancreatitis and scoliosis.[1]
Renown American actor, director, producer, and writer Christopher Reeve suffered from the acute form of SMA syndrome as a result of spinal cord injury.
Mortality
Mortality has been reported to be as high as 33%. [4] Delay in the diagnosis of SMA syndrome can result in fatal catabolysis (advanced malnutrition), dehydration, oliguria, electrolyte abnormalities, hypokalemia, acute gastric rupture or intestinal perforation (from prolonged mesenteric ischemia), gastrectasia, spontaneous upper gastrointestinal bleeding, hypovolemic shock, aspiration pneumonia, or sudden cardiovascular collapse (from increased velocity of bloodflow in the SMA due to the reduced mesenteric angle). [1][11] [12]
Diagnosis
-
Abdominal and pelvic computed tomography scan showing duodenal compression (black arrow) by the superior mesenteric artery (red arrow) and the abdominal aorta (blue arrow).
-
Upper gastrointestinal series showing extreme duodenal dilation (white arrow) abruptly preceeding constriction by the SMA.
-
A diagram of a healthy mesenteric angle.
-
A diagram of a compressed duodenum due to a reduced mesenteric angle.
Diagnosis is very difficult, and usually one of exclusion. SMA syndrome is thus considered only after patients have undergone an extensive evaluation of their gastrointestinal tract including upper endoscopy, colonoscopy, and evaluation for various malabsorptive, ulcerative and inflammatory instestinal conditions with a higher diagnostic frequency. Diagnosis may follow x-ray examination revealing duodenal dilation followed by abrupt constriction proximal to the overlying SMA, as well as a delay in transit of four to six hours through the gastroduodenal region. Suggested exams include abdominal and pelvic computed tomography (CT) scan with oral and IV contrast, and upper gastrointestinal series (UGI). Ultrasound may indicate increased bloodflow velocity through the SMA, [4] signifying risk of fatal cardiovascular or circulatory collapse. [11] Vascular imaging studies such as contrast angiography are therefore recommended.
Despite multiple case reports, there has been controversy surrounding the diagnosis and even the existence of SMA syndrome since symptoms do not always correlate well with radiologic findings, and may not always improve following surgical correction.[13] However, the reason for the persistance of gastrointestinal symptoms even after surgical correction has been recently traced to the remaining prominence of reversed peristalsis in contrast to direct peristalsis.[14]
Since females between the ages of 10 and 30 are most frequently afflicted, it is not uncommon for physicians to initially and incorrectly assume that emaciation is a choice of the patient instead of a consequence of SMA syndrome in its chronic form. Patients in the earlier stages of chronic SMA syndrome often remain unaware that they are ill until substantial damage to their health is done, since they may attempt to adapt to the condition by gradually decreasing their food intake or naturally gravitating toward a lighter and more digestible diet. Therefore, attending physicians must never conclude that symptoms are psychiatric in origin without first ordering appropriate diagnostic procedures. Eating disorder treatment protocols involving forced refeeding and behavioral therapy are noted to have poor outcomes with individuals suffering from SMA syndrome, contributing to the high mortality rate of the condition. [15]
Treatment

SMA syndrome can present in acute, acquired form (ie. abruptly emerging within an inpatient stay following scoliosis surgery) as well as chronic form (ie. developing throughout the course of a lifetime and advancing due to environmental triggers, life changes, or other illnesses). Acute cases usually respond to medical management, while chronic cases require surgical intervention.[16]
In acute or mild cases, conservative treatment should be attempted first, involving the reversal or removal of the precipitating factor with proper nutrition and replacement of fluid and electrolytes, either by surgically-inserted jejunal feeding tube, nasogastric intubation, or peripherally inserted central catheter (PICC line) administering total parenteral nutrition (TPN). Pro-motility agents such as metoclopramide (brand name "Reglan") may also be beneficial.[17] Symptoms typically improve after restoration of weight,[18] except when reversed peristalsis persists, or if regained fat refuses to accumulate within the mesenteric angle.[19]
If conservative treatment fails, or if the case is severe or chronic, surgical intervention is required. The most common operation for SMA syndrome, duodenojejunostomy, was first proposed in 1907 by Bloodgood.[6] This invasive, open surgery involves the creation of an alternate route between the duodenum and the jejunum,[20] bypassing the compression caused by the AA and the SMA.[1] Less common surgical treatments for SMA syndrome include laparoscopic, Roux-en-Y, or robotically-assisted duodenojejunostomy; gastrojejunostomy; anterior transposition of the third portion of the duodenum; intestinal derotation; and division of the ligament of Treitz. Lysis of the duodenal suspensory muscle has the advantage that it does not involve the creation of an intestinal anastomosis.[8] The world's first robotically-assisted intestinal bypass for SMA syndrome was performed on July 30, 2008 at the London Health Sciences Centre in Ontario, Canada. In contrast to traditional open duodenojejunostomy which involves a 15-centimeter scar on the upper belly, inelastic internal staples, a one-week hospital stay and significant postoperative pain, the Da Vinci robot's range of motion allows for the use of stitches in place of staples, minimizes scarring to only five one-centimeter incisions and reduces both hospital recovery time and narcotic use by more than 50%.[21]
The possible persistence of symptoms after surgical bypass can be traced to the remaining prominence of reversed peristalsis in contrast to direct peristalsis, although the precipitating factor (the duodenal compression) has been bypassed or relieved. Reversed peristalsis has been shown to respond to duodenal circular drainage—a complex, open surgical procedure originally implemented and performed in China.[22]
See also
- Catabolysis
- Malnutrition
- Medical emergency
- Median arcuate ligament syndrome
- Nutcracker syndrome
- Small bowel obstruction
- Rare diseases
- Visceroptosis
- Wasting
References
- ^ a b c d e f g h Avinash Shetty (2006-07-16). "Superior Mesenteric Artery Syndrome". eMedicine. WebMD. Retrieved 2008-04-09.
- ^ Welsch T, Büchler MW, Kienle P (2007). "Recalling superior mesenteric artery syndrome". Dig Surg. 24 (3): 149–56. doi:10.1159/000102097. PMID 17476104.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cohen LB, Field SP, Sachar DB (1985). "The superior mesenteric artery syndrome. The disease that isn't, or is it?". J. Clin. Gastroenterol. 7 (2): 113–6. doi:10.1097/00004836-198504000-00002. PMID 4008904.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Christopher T. Buresh, MD, and Mark A. Graber, MD. "Unusual Causes of Recurrent Abdominal Pain", Emerg Med 38(5):11-18, 2006. http://www.emedmag.com/html/pre/fea/features/051506.asp
- ^ a b Laffont I, Bensmail D, Rech C, Prigent G, Loubert G, Dizien O (2002). "Late superior mesenteric artery syndrome in paraplegia: case report and review". Spinal Cord. 40 (2): 88–91. doi:10.1038/sj.sc.3101255. PMID 11926421.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Baltazar U, Dunn J, Floresguerra C, Schmidt L, Browder W (2000). "Superior mesenteric artery syndrome: an uncommon cause of intestinal obstruction". South. Med. J. 93 (6): 606–8. PMID 10881780.
{{cite journal}}: CS1 maint: multiple names: authors list (link)Free full text with registration at Medscape - ^ "S: Superior mesenteric artery syndrome". GASTROLAB Digestive Dictionary. GASTROLAB. April 1, 2008. Retrieved 2008-04-09.
- ^ a b http://www.cma.ca/index.cfm/ci_id/35163/la_id/1.htm
- ^ http://journals.lww.com/spinejournal/Abstract/2002/12150/Superior_Mesenteric_Artery_Syndrome_Following.23.aspx
- ^ Hoffman, Robert J. and Stephen M. Arpadi. "Case Report: A Pediatric AIDS Patient with Superior Mesenteric Artery Syndrome." http://www.liebertonline.com/doi/abs/10.1089/108729100318073
- ^ a b Errico, Thomas J. "Surgical Management of Spinal Deformities", 458
- ^ Kai-Hsiung Ko; Shih-Hung Tsai; Chih-Yung Yu; Guo-Shu Huang; Chang-Hsien Liu; Wei-Chou Chang. http://www.biomedsearch.com/nih/Unusual-complication-superior-mesenteric-artery/19181598.html"
- ^ http://www.uptodate.com/patients/content/topic.do?print=true&topicKey=gi_dis/31570&view=print
- ^ Yang, Wei-Liang and Xin-Chen Zhang. World Journal of Gastroenterology 2008 January 14; 14(2): 303-306 http://www.wjgnet.com/1007-9327/14/303.pdf
- ^ Schellenberg, Randy. "Medical Disorders And Conditions That Can Cause Anorexia, Weight Loss, Or Vomiting". http://randyschellenberg.tripod.com/anorexiatruthinfo/id1.html
- ^ http://everything2.com/title/Superior+mesenteric+artery+syndrome
- ^ http://everything2.com/title/Superior+mesenteric+artery+syndrome
- ^ Manu N, Martin L (2006). "Weight Loss Induced Small Bowel Obstruction". The Internet Journal of Gastroenterology. 4 (2).
- ^ Yang, Wei-Liang and Xin-Chen Zhang. World Journal of Gastroenterology 2008 January 14; 14(2): 303-306 http://www.wjgnet.com/1007-9327/14/303.pdf
- ^ "Duodenojejunostomy". The Free Dictionary. Farlex. Retrieved 2008-04-09.
- ^ http://www.lhsc.on.ca/About_Us/CSTAR/News/SMA.htm
- ^ Yang, Wei-Liang and Xin-Chen Zhang. World Journal of Gastroenterology 2008 January 14; 14(2): 303-306 http://www.wjgnet.com/1007-9327/14/303.pdf