Autoinducer-2: Difference between revisions
More on this signalling |
use citation templates |
||
| Line 7: | Line 7: | ||
}} |
}} |
||
'''Autoinducer-2''' (AI-2), a furanosyl borate diester, is a member of a family of [[signaling molecule]]s used in [[quorum sensing]].<ref>Cao |
'''Autoinducer-2''' (AI-2), a furanosyl borate diester, is a member of a family of [[signaling molecule]]s used in [[quorum sensing]].<ref>{{cite journal | last1= Cao | first1= J.G. | last2= Meighen | first2= E.A. | year= 1989 | title= | journal= [[Journal of Biological Chemistry]] | volume= 264 | series= | issue= | pages= 21670-21676 | issn= | pmid= | pmc= | doi= | bibcode= | oclc= | id= | url= | accessdate= [[2010-03-08]] }}</ref><ref>{{cite journal | last1= Miller | first1= S.T. | year= 2004 | title= | journal= [[Molecular Cell]] | volume= 15 | series= | issue= | pages= 677-687 | issn= | pmid= | pmc= | doi= | bibcode= | oclc= | id= | url= | accessdate= [[2010-03-08]] }}</ref> AI-2 is produced by both [[gram-negative]] and [[gram-positive bacteria]].<ref>{{cite journal | last1= Miller | first1= M. B. | last2= Bassler | first2= B. L. | year= 2001 | title= Quorum sensing in bacteria | journal= [[Annual Review of Microbiology]] | volume= 55 | series= | issue= | pages= 165-199 | pmid= 11544353 | accessdate= [[2010-03-08]] }}</ref> AI-2 is sythesized by the reaction of 1-deoxy-3-dehydro-D-[[ribulose]] with [[boric acid]].<ref>http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/misc/AI2.html</ref> |
||
AI-2 is sensed by the Lsr transport cassette and is actively transported into the cell, where it is phosphorylated by LsrK. Then, Phospho-AI-2 binds the transcriptional repressor protein, LsrR, which subsequently is released from the promoter/operator region of the lsr operon – and transcription of the lsr genes is initiated. AI-2 signalling is also regulated by glucose and cAMP/CRP via the lsr operon. In the presence of glucose, low levels of cAMP/CRP result in almost no lsr operon (lsrABCDFG) expression. Without glucose, cAMP-CRP is needed to stimulate the lsr expression, while LsrR represses its expression in the absence of the inducer, phospho-AI-2. As AI-2 accumulates, more AI-2 is taken in via LsrABCD, phosphorylated via LsrK, and the lsr transcription is de-repressed, enabling even more AI-2 uptake.<ref>Wang |
AI-2 is sensed by the Lsr transport cassette and is actively transported into the cell, where it is phosphorylated by LsrK. Then, Phospho-AI-2 binds the transcriptional repressor protein, LsrR, which subsequently is released from the promoter/operator region of the lsr operon – and transcription of the lsr genes is initiated. AI-2 signalling is also regulated by glucose and cAMP/CRP via the lsr operon. In the presence of glucose, low levels of cAMP/CRP result in almost no lsr operon (lsrABCDFG) expression. Without glucose, cAMP-CRP is needed to stimulate the lsr expression, while LsrR represses its expression in the absence of the inducer, phospho-AI-2. As AI-2 accumulates, more AI-2 is taken in via LsrABCD, phosphorylated via LsrK, and the lsr transcription is de-repressed, enabling even more AI-2 uptake.<ref>{{cite journal | last1= Wang | first1= L. | year= 2005 | title= | journal= [[Journal of Bacteriology]] | volume= 187 | series= | issue= | pages= 2066-2076 | issn= | pmid= | pmc= | doi= | bibcode= | oclc= | id= | url= | accessdate= [[2010-03-08]] }}</ref> |
||
==References== |
==References== |
||
Revision as of 20:59, 8 March 2010
| |
| Names | |
|---|---|
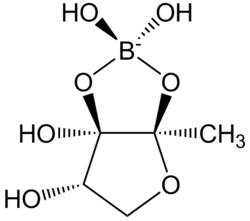
| IUPAC name
(3aS,6S,6aR)-2,2,6,6a-tetrahydroxy-3a-methyltetrahydrofuro[3,2-d][1,3,2]dioxaborol-2-uide)
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
Autoinducer-2 (AI-2), a furanosyl borate diester, is a member of a family of signaling molecules used in quorum sensing.[1][2] AI-2 is produced by both gram-negative and gram-positive bacteria.[3] AI-2 is sythesized by the reaction of 1-deoxy-3-dehydro-D-ribulose with boric acid.[4]
AI-2 is sensed by the Lsr transport cassette and is actively transported into the cell, where it is phosphorylated by LsrK. Then, Phospho-AI-2 binds the transcriptional repressor protein, LsrR, which subsequently is released from the promoter/operator region of the lsr operon – and transcription of the lsr genes is initiated. AI-2 signalling is also regulated by glucose and cAMP/CRP via the lsr operon. In the presence of glucose, low levels of cAMP/CRP result in almost no lsr operon (lsrABCDFG) expression. Without glucose, cAMP-CRP is needed to stimulate the lsr expression, while LsrR represses its expression in the absence of the inducer, phospho-AI-2. As AI-2 accumulates, more AI-2 is taken in via LsrABCD, phosphorylated via LsrK, and the lsr transcription is de-repressed, enabling even more AI-2 uptake.[5]
References
- ^ Cao, J.G.; Meighen, E.A. (1989). Journal of Biological Chemistry. 264: 21670–21676.
{{cite journal}}:|access-date=requires|url=(help); Check date values in:|accessdate=(help); Missing or empty|title=(help) - ^ Miller, S.T. (2004). Molecular Cell. 15: 677–687.
{{cite journal}}:|access-date=requires|url=(help); Check date values in:|accessdate=(help); Missing or empty|title=(help) - ^ Miller, M. B.; Bassler, B. L. (2001). "Quorum sensing in bacteria". Annual Review of Microbiology. 55: 165–199. PMID 11544353.
{{cite journal}}:|access-date=requires|url=(help); Check date values in:|accessdate=(help) - ^ http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/misc/AI2.html
- ^ Wang, L. (2005). Journal of Bacteriology. 187: 2066–2076.
{{cite journal}}:|access-date=requires|url=(help); Check date values in:|accessdate=(help); Missing or empty|title=(help)
