Nuclear fission: Difference between revisions
m Reverted edits by 173.12.26.213 to last revision by Bradjamesbrown (HG) |
|||
| Line 40: | Line 40: | ||
The total rest masses of the fission products ('''Mp''') from a single reaction is less than the mass of the original fuel nucleus ('''M'''). The excess mass '''Δm''' = '''M''' – '''Mp''' is the [[invariant mass]] of the energy that is released as [[photon]]s ([[gamma ray]]s) and kinetic energy of the fission fragments, according to the [[mass-energy equivalence]] formula ''E'' = ''mc''². |
The total rest masses of the fission products ('''Mp''') from a single reaction is less than the mass of the original fuel nucleus ('''M'''). The excess mass '''Δm''' = '''M''' – '''Mp''' is the [[invariant mass]] of the energy that is released as [[photon]]s ([[gamma ray]]s) and kinetic energy of the fission fragments, according to the [[mass-energy equivalence]] formula ''E'' = ''mc''². |
||
The variation in specific binding energy with [[atomic number]] is due to the interplay of the two fundamental [[force]]s acting on the component [[nucleon]]s ([[proton]]s and [[neutron]]s) that make up the nucleus. Nuclei are bound by an attractive [[nuclear force]] between nucleons, which overcomes the [[electrostatic repulsion]] between protons. However, the nuclear force acts only over relatively short ranges (a few [[nucleon]] diameters), since it follows an exponentially decaying [[Yukawa potential]] which makes it insignificant at longer distances. The electrostatic repulsion is of longer range, since it decays by an inverse-square rule, so that nuclei larger than about 12 nucleons in diameter reach a point that the total electrostatic repulsion overcomes the nuclear force and causes them to be spontaneously unstable. For the same reason, larger nuclei (more than about eight nucleons in diameter) are less tightly bound per unit mass than are smaller nuclei; breaking a large nucleus into two or more intermediate-sized nuclei, releases energy. The origin of this energy is the nuclear force, which intermediate-sized nuclei allows to act more efficiently, because each nucleon has more neighbors which are within the short range attraction of this force. |
|||
The variation in specific binding energy with [[atomic number]] is due to the interplay of the two fundamental [[force]]s acting on the component [[nucleon]]s ([[protonneighbors which are within the short range attraction of this force. |
|||
Also because of the short range of the strong binding force, large stable nuclei must contain proportionally more neutrons than do the lightest elements, which are most stable with a '''1 to 1 ratio''' of protons and neutrons. Nuclei which have more than 20 protons cannot be stable unless they have more than an equal number of neutrons. Extra neutrons stabilize heavy elements because they add to strong-force binding (which acts between all nucleons), without adding to proton-proton repulsion. Fission products have, on |
Also because of the short range of the strong binding force, large stable nuclei must contain proportionally more neutrons than do the lightest elements, which are most stable with a '''1 to 1 ratio''' of protons and neutrons. Nuclei which have more than 20 protons cannot be stable unless they have more than an equal number of neutrons. Extra neutrons stabilize heavy elements because they add to strong-force binding (which acts between all nucleons), without adding to proton-proton repulsion. Fission products have, on average, about the same ratio of neutrons and protons as their parent nucleus, and are therefore usually unstable to beta decay (which changes neutrons to protons) because they have proportionally too many neutrons compared to stable isotopes of similar mass. This tendency for fission product nuclei to beta-decay is the fundamental cause of the problem of [[radioactive]] [[high level waste]] from nuclear reactors. Fission products tend to be [[beta ray|beta emitters]], [[beta decay|emitting]] fast-moving [[electron]]s to conserve [[electric charge]], as excess neutrons convert to protons in the fission-product atoms. |
||
=== Nuclear or "fissile" fuels === |
=== Nuclear or "fissile" fuels === |
||
Revision as of 18:44, 26 March 2010
- "Splitting the atom" redirects here. For the EP of the same name by Massive Attack, see Splitting the Atom.
| Nuclear physics |
|---|
 |

In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts, often producing free neutrons and lighter nuclei, which may eventually produce photons (in the form of gamma rays). Fission of heavy elements is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). For fission to produce energy, the total binding energy of the resulting elements has to be higher than that of the starting element. Fission is a form of nuclear transmutation because the resulting fragments are not the same element as the original atom.
Nuclear fission produces energy for nuclear power and to drive the explosion of nuclear weapons. Both uses are made possible because certain substances called nuclear fuels undergo fission when struck by free neutrons and in turn generate neutrons when they break apart. This makes possible a self-sustaining chain reaction that releases energy at a controlled rate in a nuclear reactor or at a very rapid uncontrolled rate in a nuclear weapon.
The amount of free energy contained in nuclear fuel is millions of times the amount of free energy contained in a similar mass of chemical fuel such as gasoline, making nuclear fission a very tempting source of energy; however, the products of nuclear fission are radioactive and remain so for significant amounts of time, giving rise to a nuclear waste problem. Concerns over nuclear waste accumulation and over the destructive potential of nuclear weapons may counterbalance the desirable qualities of fission as an energy source, and give rise to ongoing political debate over nuclear power.
Physical overview
Mechanics
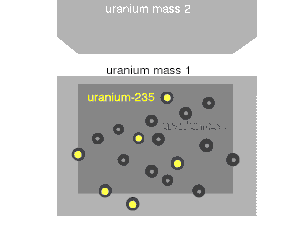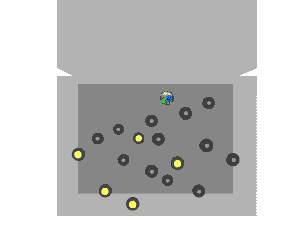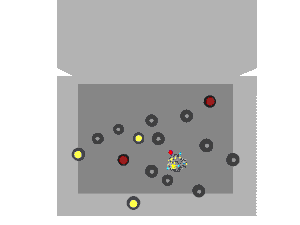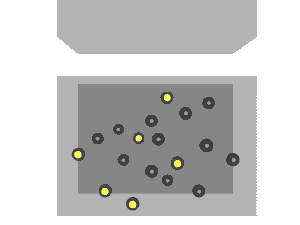
Nuclear fission can occur without neutron bombardment, as a type of radioactive decay. This type of fission (called spontaneous fission) is rare except in a few heavy isotopes. In engineered nuclear devices, essentially all nuclear fission occurs as a "nuclear reaction"—a bombardment-driven process that results from the collision of two subatomic particles. In nuclear reactions, a subatomic particle collides with an atomic nucleus and causes changes to it. Nuclear reactions are thus driven by the mechanics of bombardment, not by the relatively constant exponential decay and half-life characteristic of spontaneous radioactive processes.
A great amount of nuclear reactions are known. Nuclear fission differs importantly from other types of nuclear reactions in that it can be amplified and sometimes controlled via a nuclear chain reaction. In such a reaction, free neutrons released by each fission event can trigger yet more events, which in turn release more neutrons and cause more fissions.
The chemical element isotopes that can sustain a fission chain reaction are called nuclear fuels, and are said to be fissile. The most common nuclear fuels are 235U (the isotope of uranium with an atomic mass of 235 and of use in nuclear reactors) and 239Pu (the isotope of plutonium with an atomic mass of 239). These fuels break apart into a bimodal range of chemical elements with atomic masses centering near 95 and 135 u (fission products). Most nuclear fuels undergo spontaneous fission only very slowly, decaying instead mainly via an alpha/beta decay chain over periods of millennia to eons. In a nuclear reactor or nuclear weapon, the overwhelming majority of fission events are induced by bombardment with another particle, a neutron, which is itself produced by prior fission events.
Energetics
Typical fission events release about two hundred million eV of energy for each fission event. By contrast, most chemical oxidation reactions (such as burning coal or TNT) release at most a few eV per event, so nuclear fuel contains at least ten million times more usable energy than does chemical fuel. The energy of nuclear fission is released as kinetic energy of the fission products and fragments, and as electromagnetic radiation in the form of gamma rays; in a nuclear reactor, the energy is converted to heat as the particles and gamma rays collide with the atoms that make up the reactor and its working fluid, usually water or occasionally heavy water.
When a uranium nucleus fissions into two daughter nuclei fragments, an energy of ~200 MeV is released. For uranium-235 (total mean fission energy 202.5 MeV), typically ~169 MeV appears as the kinetic energy of the daughter nuclei, which fly apart at about 3% of the speed of light, due to Coulomb repulsion. Also, an average of 2.5 neutrons are emitted with a kinetic energy of ~2 MeV each (total of 4.8 MeV). The fission reaction also releases ~7 MeV in prompt gamma ray photons. The latter figure means that a nuclear explosion or criticality accident emits about 3.5% of its energy as gamma rays, less than 2.5% of its energy as fast neutrons, and the rest as kinetic energy of fission fragments ("heat"). In an atomic bomb, this heat may serve to raise the temperature of the bomb core to 100 million kelvins and cause secondary emission of soft X-rays, which convert some of this energy to ionizing radiation. However, in nuclear reactors, the fission fragment kinetic energy remains as low-temperature heat which causes little or no ionization.
The total prompt fission energy amounts to about 181 MeV, or ~ 89% of the total energy. The remaining ~ 11% is released in beta decays which have various half-lives, but begin as a process in the fission products immediately; and in delayed gamma emissions associated with these beta decays. For example, in uranium-235 this delayed energy is divided into about 6.5 MeV in betas, 8.8 MeV in antineutrinos (released at the same time as the betas), and finally, an additional 6.3 MeV in delayed gamma emission from the excited beta-decay products (for a mean total of ~ 10 gamma ray emissions per fission, in all).
The 8.8 MeV/202.5 MeV = 4.3% of the energy which is released as antineutrinos is not captured by the reactor material as heat, and escapes directly through all materials (including the Earth) at nearly the speed of light, and into interplanetary space. Almost all of the remaining radiation is converted to heat, either in the reactor core or its shielding.
Some processes involving neutrons are notable for absorbing or finally yielding energy—for example neutron kinetic energy does not yield heat immediately if the neutron is captured by a uranium-238 atom to breed plutonium-239, but this energy is emitted if the plutonium-239 is later fissioned. On the other hand, so called "delayed neutrons" emitted as radioactive decay products with half-lives up to a minute, from fission-daughters, are very important to reactor control because they give a characteristic "reaction" time for the total nuclear reaction to double in size, if the reaction is run in a "delayed-critical" zone which deliberately relies on these neutrons for a supercritical chain-reaction (one in which each fission cycle yields more neutrons than it absorbs). Without their existence, the nuclear chain-reaction would be prompt critical and increase in size faster than it could be controlled by human intervention. In this case, the first experimental atomic reactors would have run away to a dangerous and messy "prompt critical reaction" before their operators could have manually shut them down (for this reason, designer Enrico Fermi included radiation-counter-triggered control rods, suspended by electromagnets, which could automatically drop into the center of Chicago Pile-1). If these delayed neutrons are captured without producing fissions, they produce heat as well.[1]
Product nuclei and binding energy
In fission there is a preference to yield fragments with even proton numbers, which is called the odd-even effect on the fragments charge distribution. However, no odd-even effect is observed on fragment mass number distribution. This result is attributed to nucleon pair breaking.
In nuclear fission events the nuclei may break into any combination of lighter nuclei, but the most common event is not fission to equal mass nuclei of about mass 120; the most common event (depending on isotope and process) is a slightly unequal fission in which one daughter nucleus has a mass of about 90 to 100 u and the other the remaining 130 to 140 u.[2] Unequal fissions are energetically more favorable because this allows one product to be closer to the energetic minimum near mass 60 u (only a quarter of the average fissionable mass), while the other nucleus with mass 135 u is still not far out of the range of the most tightly bound nuclei (another statement of this, is that the atomic binding energy curve is slightly steeper to the left of mass 120 u than to the right of it).
Origin of the active energy and the curve of binding energy
Nuclear fission of heavy elements produces energy because the specific binding energy (binding energy per mass) of intermediate-mass nuclei with atomic numbers and atomic masses close to 62Ni and 56Fe is greater than the nucleon-specific binding energy of very heavy nuclei, so that energy is released when heavy nuclei are broken apart.
The total rest masses of the fission products (Mp) from a single reaction is less than the mass of the original fuel nucleus (M). The excess mass Δm = M – Mp is the invariant mass of the energy that is released as photons (gamma rays) and kinetic energy of the fission fragments, according to the mass-energy equivalence formula E = mc². The variation in specific binding energy with atomic number is due to the interplay of the two fundamental forces acting on the component nucleons (protons and neutrons) that make up the nucleus. Nuclei are bound by an attractive nuclear force between nucleons, which overcomes the electrostatic repulsion between protons. However, the nuclear force acts only over relatively short ranges (a few nucleon diameters), since it follows an exponentially decaying Yukawa potential which makes it insignificant at longer distances. The electrostatic repulsion is of longer range, since it decays by an inverse-square rule, so that nuclei larger than about 12 nucleons in diameter reach a point that the total electrostatic repulsion overcomes the nuclear force and causes them to be spontaneously unstable. For the same reason, larger nuclei (more than about eight nucleons in diameter) are less tightly bound per unit mass than are smaller nuclei; breaking a large nucleus into two or more intermediate-sized nuclei, releases energy. The origin of this energy is the nuclear force, which intermediate-sized nuclei allows to act more efficiently, because each nucleon has more neighbors which are within the short range attraction of this force.
Also because of the short range of the strong binding force, large stable nuclei must contain proportionally more neutrons than do the lightest elements, which are most stable with a 1 to 1 ratio of protons and neutrons. Nuclei which have more than 20 protons cannot be stable unless they have more than an equal number of neutrons. Extra neutrons stabilize heavy elements because they add to strong-force binding (which acts between all nucleons), without adding to proton-proton repulsion. Fission products have, on average, about the same ratio of neutrons and protons as their parent nucleus, and are therefore usually unstable to beta decay (which changes neutrons to protons) because they have proportionally too many neutrons compared to stable isotopes of similar mass. This tendency for fission product nuclei to beta-decay is the fundamental cause of the problem of radioactive high level waste from nuclear reactors. Fission products tend to be beta emitters, emitting fast-moving electrons to conserve electric charge, as excess neutrons convert to protons in the fission-product atoms.
Nuclear or "fissile" fuels
The most common nuclear fuels, 235U and 239Pu, are not major radiological hazards by themselves: 235U has a half-life of approximately 700 million years, and although 239Pu has a half-life of only about 24,000 years, it is a pure alpha particle emitter and hence not particularly dangerous unless ingested. Once a fuel element has been used, the remaining fuel material is intimately mixed with highly radioactive fission products that emit energetic beta particles and gamma rays. Some fission products have half-lives as short as seconds; others have half-lives of tens of thousands of years, requiring long-term storage in facilities such as Yucca Mountain nuclear waste repository until the fission products decay into non-radioactive stable isotopes.
Chain reactions

Several heavy elements, such as uranium, thorium, and plutonium, undergo both spontaneous fission, a form of radioactive decay and induced fission, a form of nuclear reaction. Elemental isotopes that undergo induced fission when struck by a free neutron are called fissionable; isotopes that undergo fission when struck by a thermal, slow moving neutron are also called fissile. A few particularly fissile and readily obtainable isotopes (notably 235U and 239Pu) are called nuclear fuels because they can sustain a chain reaction and can be obtained in large enough quantities to be useful.
All fissionable and fissile isotopes undergo a small amount of spontaneous fission which releases a few free neutrons into any sample of nuclear fuel. Such neutrons would escape rapidly from the fuel and become a free neutron, with a mean lifetime of about 15 minutes before decaying to protons and beta particles. However, neutrons almost invariably impact and are absorbed by other nuclei in the vicinity long before this happens (newly-created fission neutrons move at about 7% of the speed of light, and even moderated neutrons move at about 8 times the speed of sound). Some neutrons will impact fuel nuclei and induce further fissions, releasing yet more neutrons. If enough nuclear fuel is assembled in one place, or if the escaping neutrons are sufficiently contained, then these freshly generated neutrons outnumber the neutrons that escape from the assembly, and a sustained nuclear chain reaction will take place.
An assembly that supports a sustained nuclear chain reaction is called a critical assembly or, if the assembly is almost entirely made of a nuclear fuel, a critical mass. The word "critical" refers to a cusp in the behavior of the differential equation that governs the number of free neutrons present in the fuel: if less than a critical mass is present, then the amount of neutrons is determined by radioactive decay, but if a critical mass or more is present, then the amount of neutrons is controlled instead by the physics of the chain reaction. The actual mass of a critical mass of nuclear fuel depends strongly on the geometry and surrounding materials.
Not all fissionable isotopes can sustain a chain reaction. For example, 238U, the most abundant form of uranium, is fissionable but not fissile: it undergoes induced fission when impacted by an energetic neutron with over 1 MeV of kinetic energy. But too few of the neutrons produced by 238U fission are energetic enough to induce further fissions in 238U, so no chain reaction is possible with this isotope. Instead, bombarding 238U with slow neutrons causes it to absorb them (becoming 239U) and decay by beta emission to 239Np which then decays again by the same process to 239Pu; that process is used to manufacture 239Pu in breeder reactors. In-situ plutonium production also contributes to the neutron chain reaction in other types of reactors after sufficient plutonium-239 has been produced, since plutonium-239 is also a fissile element which serves as fuel. It is estimated that up to half of the power produced by a standard "non-breeder" reactor is produced by the fission of plutonium-239 produced in place, over the total life-cycle of a fuel load.
Fissionable, non-fissile isotopes can be used as fission energy source even without a chain reaction. Bombarding 238U with fast neutrons induces fissions, releasing energy as long as the external neutron source is present. This is an important effect in all reactors where fast neutrons from the fissile isotope can cause the fission of nearby 238U nuclei, which means that some small part of the 238U is "burned-up" in all nuclear fuels, especially in fast breeder reactors that operate with higher-energy neutrons. That same fast-fission effect is used to augment the energy released by modern thermonuclear weapons, by jacketing the weapon with 238U to react with neutrons released by nuclear fusion at the center of the device.
Fission reactors
Critical fission reactors are the most common type of nuclear reactor. In a critical fission reactor, neutrons produced by fission of fuel atoms are used to induce yet more fissions, to sustain a controllable amount of energy release. Devices that produce engineered but non-self-sustaining fission reactions are subcritical fission reactors. Such devices use radioactive decay or particle accelerators to trigger fissions.
Critical fission reactors are built for three primary purposes, which typically involve different engineering trade-offs to take advantage of either the heat or the neutrons produced by the fission chain reaction:
- power reactors are intended to produce heat for nuclear power, either as part of a generating station or a local power system such as a nuclear submarine.
- research reactors are intended to produce neutrons and/or activate radioactive sources for scientific, medical, engineering, or other research purposes.
- breeder reactors are intended to produce nuclear fuels in bulk from more abundant isotopes. The better known fast breeder reactor makes 239Pu (a nuclear fuel) from the naturally very abundant 238U (not a nuclear fuel). Thermal breeder reactors previously tested using 232Th to breed the fissile isotope 233U continue to be studied and developed.
While, in principle, all fission reactors can act in all three capacities, in practice the tasks lead to conflicting engineering goals and most reactors have been built with only one of the above tasks in mind. (There are several early counter-examples, such as the Hanford N reactor, now decommissioned). Power reactors generally convert the kinetic energy of fission products into heat, which is used to heat a working fluid and drive a heat engine that generates mechanical or electrical power. The working fluid is usually water with a steam turbine, but some designs use other materials such as gaseous helium. Research reactors produce neutrons that are used in various ways, with the heat of fission being treated as an unavoidable waste product. Breeder reactors are a specialized form of research reactor, with the caveat that the sample being irradiated is usually the fuel itself, a mixture of 238U and 235U. For a more detailed description of the physics and operating principles of critical fission reactors, see nuclear reactor physics. For a description of their social, political, and environmental aspects, see nuclear reactor.
Fission bombs

One class of nuclear weapon, a fission bomb (not to be confused with the fusion bomb), otherwise known as an atomic bomb or atom bomb, is a fission reactor designed to liberate as much energy as possible as rapidly as possible, before the released energy causes the reactor to explode (and the chain reaction to stop). Development of nuclear weapons was the motivation behind early research into nuclear fission: the Manhattan Project of the U.S. military during World War II carried out most of the early scientific work on fission chain reactions, culminating in the Trinity test bomb and the Little Boy and Fat Man bombs that were exploded over the cities Hiroshima, and Nagasaki, Japan in August 1945.
Even the first fission bombs were thousands of times more explosive than a comparable mass of chemical explosive. For example, Little Boy weighed a total of about four tons (of which 60 kg was nuclear fuel) and was 11 feet (3.4 m) long; it also yielded an explosion equivalent to about 15 kilotons of TNT, destroying a large part of the city of Hiroshima. Modern nuclear weapons (which include a thermonuclear fusion as well as one or more fission stages) are literally hundreds of times more energetic for their weight than the first pure fission atomic bombs, so that a modern single missile warhead bomb weighing less than 1/8th as much as Little Boy (see for example W88) has a yield of 475,000 tons of TNT, and could bring destruction to 10 times the city area.
While the fundamental physics of the fission chain reaction in a nuclear weapon is similar to the physics of a controlled nuclear reactor, the two types of device must be engineered quite differently (see nuclear reactor physics). It is impossible to convert a nuclear reactor to cause a true nuclear explosion[citation needed], or for a nuclear reactor to explode the way a nuclear explosive does, (though partial fuel meltdowns and steam explosions have occurred). It is also difficult to extract useful power from a nuclear explosive, although at least one rocket propulsion system, Project Orion, is intended to work by exploding fission bombs behind a massively-padded and shielded vehicle.
The strategic importance of nuclear weapons is a major reason why the technology of nuclear fission is politically sensitive. Viable fission bomb designs are, arguably, within the capabilities of bright undergraduates (see John Aristotle Phillips) being relatively simple from an engineering viewpoint. However, the difficulty of obtaining fissile nuclear material to realize the designs, is the key to the relative unavailability of nuclear weapons to all but modern industrialized governments with special programs to produce fissile materials (see uranium enrichment and nuclear fuel cycle).
History
Natural fission chain-reactors on Earth
Criticality in nature is uncommon. At three ore deposits at Oklo in Gabon, sixteen sites (the so-called Oklo Fossil Reactors) have been discovered at which self-sustaining nuclear fission took place approximately 2 billion years ago. Unknown until 1972 (but postulated by Paul Kuroda in 1956[3]), when French physicist Francis Perrin discovered the Oklo Fossil Reactors, it was realized that nature had beaten humans to the punch. Large-scale natural uranium fission chain reactions, moderated by normal water, had occurred far in the past and would not be possible now. This ancient process was able to use normal water as a moderator only because 2 billion years before the present, natural uranium was richer in the shorter-lived fissile isotope 235U (about 3%), than natural uranium available today (which is only 0.7%, and must be enriched to 3% to be usable in light-water reactors).
Artificial nuclear fission
New Zealander, Ernest Rutherford is credited with splitting the atom in 1917.[4] His team in Manchester, England bombarded nitrogen with naturally occurring alpha particles from radioactive material and observed a proton emitted with energy higher than the alpha particle. In 1932 his students John Cockcroft and Ernest Walton, working under Rutherford's direction, attempted to split the nucleus by entirely artificial means, using a particle accelerator to bombard lithium with protons, thereby producing two helium nuclei.[5]
After English physicist James Chadwick discovered the neutron in 1932,[6] Enrico Fermi and his colleagues in Rome studied the results of bombarding uranium with neutrons in 1934.[7] The first person who mentioned the idea of nuclear fission in 1934 was Ida Noddack.[8][9]

After the Fermi publication, Lise Meitner, Otto Hahn and Fritz Strassmann began performing similar experiments in Germany. Meitner, an Austrian Jew, lost her citizenship with the Anschluss in 1938. She fled and wound up in Sweden, but continued to collaborate by mail and through meetings with Hahn in Sweden. By coincidence her nephew Otto Robert Frisch, also a refugee, was also in Sweden when Meitner received a letter from Hahn describing his chemical proof that some of the product of the bombardment of uranium with neutrons, was barium and not barium's much heavier chemical sister element radium (barium's atomic weight is about 60% that of uranium). Frisch was skeptical, but Meitner trusted Hahn's ability as a chemist. Marie Curie had been separating barium from radium for many years, and the techniques were well-known. According to Frisch:
Was it a mistake? No, said Lise Meitner; Hahn was too good a chemist for that. But how could barium be formed from uranium? No larger fragments than protons or helium nuclei (alpha particles) had ever been chipped away from nuclei, and to chip off a large number not nearly enough energy was available. Nor was it possible that the uranium nucleus could have been cleaved right across. A nucleus was not like a brittle solid that can be cleaved or broken; George Gamow had suggested early on, and Bohr had given good arguments that a nucleus was much more like a liquid drop. Perhaps a drop could divide itself into two smaller drops in a more gradual manner, by first becoming elongated, then constricted, and finally being torn rather than broken in two? We knew that there were strong forces that would resist such a process, just as the surface tension of an ordinary liquid drop tends to resist its division into two smaller ones. But nuclei differed from ordinary drops in one important way: they were electrically charged, and that was known to counteract the surface tension.
The charge of a uranium nucleus, we found, was indeed large enough to overcome the effect of the surface tension almost completely; so the uranium nucleus might indeed resemble a very wobbly unstable drop, ready to divide itself at the slightest provocation, such as the impact of a single neutron. But there was another problem. After separation, the two drops would be driven apart by their mutual electric repulsion and would acquire high speed and hence a very large energy, about 200 MeV in all; where could that energy come from? ...Lise Meitner... worked out that the two nuclei formed by the division of a uranium nucleus together would be lighter than the original uranium nucleus by about one-fifth the mass of a proton. Now whenever mass disappears energy is created, according to Einstein's formula E=mc2, and one-fifth of a proton mass was just equivalent to 200MeV. So here was the source for that energy; it all fitted![10]
In December 1938, the German chemists Otto Hahn and Fritz Strassmann sent a manuscript to Naturwissenschaften reporting they had detected the element barium after bombarding uranium with neutrons;[11] simultaneously, they communicated these results to Lise Meitner. Meitner, and her nephew Otto Robert Frisch, correctly interpreted these results as being nuclear fission.[12] Frisch confirmed this experimentally on 13 January 1939.[13] In 1944, Hahn received the Nobel Prize for Chemistry for the discovery of nuclear fission. Some historians who have documented the history of the discovery of nuclear fission believe Meitner should have been awarded the Nobel Prize with Hahn.[14][15][16]
Meitner’s and Frisch’s interpretation of the work of Hahn and Strassmann crossed the Atlantic Ocean with Niels Bohr, who was to lecture at Princeton University. Isidor Isaac Rabi and Willis Lamb, two Columbia University physicists working at Princeton, heard the news and carried it back to Columbia. Rabi said he told Enrico Fermi; Fermi gave credit to Lamb. Bohr soon thereafter went from Princeton to Columbia to see Fermi. Not finding Fermi in his office, Bohr went down to the cyclotron area and found Herbert L. Anderson. Bohr grabbed him by the shoulder and said: “Young man, let me explain to you about something new and exciting in physics.”[17] It was clear to a number of scientists at Columbia that they should try to detect the energy released in the nuclear fission of uranium from neutron bombardment. On 25 January 1939, a Columbia University team conducted the first nuclear fission experiment in the United States,[18] which was done in the basement of Pupin Hall; the members of the team were Herbert L. Anderson, Eugene T. Booth, John R. Dunning, Enrico Fermi, G. Norris Glasoe, and Francis G. Slack. The next day, the Fifth Washington Conference on Theoretical Physics began in Washington, D.C. under the joint auspices of the George Washington University and the Carnegie Institution of Washington. There, the news on nuclear fission was spread even further, which fostered many more experimental demonstrations.[19]
Frédéric Joliot-Curie's team in Paris discovered that secondary neutrons are released during uranium fission, thus making a nuclear chain-reaction feasible. The figure of about two neutrons being emitted with nuclear fission of uranium was verified independently by Leo Szilárd and Walter Henry Zinn. The number of neutrons emitted with nuclear fission of 235U was then reported at 3.5/fission, and later corrected to 2.6/fission by Frédéric Joliot-Curie, Hans von Halban and Lew Kowarski.
The fission chain reaction
"Chain reactions" at that time were a known phenomenon in chemistry, but the analogous process in nuclear physics, using neutrons, had been foreseen as early as 1933 by Szilárd, although Szilárd at that time had no idea with what materials the process might be initiated (Szilárd thought it might be done with light neutron-rich elements). Szilárd, a Hungarian born Jew, also fled mainland Europe after Hitler's rise, eventually landing in the US.
With the news of fission neutrons from uranium fission, Szilárd immediately understood the possibility of a nuclear chain reaction using uranium. In the summer, Fermi and Szilard proposed the idea of a nuclear reactor (pile) to mediate this process. The pile would use natural uranium as fuel, and graphite as the moderator of neutron energy (it had previously been shown by Fermi that neutrons were far more effectively captured by atoms if they were moving slowly, a process called moderation when the neutrons were slowed after being released from a fission event in a nuclear reactor).
In August Hungarian-Jewish refugees Szilard, Teller and Wigner thought that the Germans might make use of the fission chain reaction. They decided to warn President Roosevelt of this possible German menace, and persuaded German-Jewish refugee Albert Einstein to lend his name. The Einstein–Szilárd letter suggested the possibility of a uranium bomb deliverable by ship, which would destroy "an entire harbor and much of the surrounding countryside." The President received the letter on 11 October 1939 — shortly after World War II began in Europe, but two years before U.S. entry into it.
In England, James Chadwick proposed an atomic bomb utilizing natural uranium, based on a paper by Rudolf Peierls with the mass needed for critical state being 30–40 tons. In America, J. Robert Oppenheimer thought that a cube of uranium deuteride 10 cm on a side (about 11 kg of uranium) might "blow itself to hell." In this design it was still thought that a moderator would need to be used for nuclear bomb fission (this turned out not to be the case if the fissile isotope was separated).
In December, Heisenberg delivered a report to the German Ministry of War on the possibility of a uranium bomb.
In Birmingham, England, Frisch teamed up with Peierls, a fellow German-Jewish refugee. They had the idea of using a purified mass of the uranium isotope 235U, which had a cross section just determined, and which was much larger than that of 238U or natural uranium (which is 99.3% the latter isotope). Assuming that the cross section for fast-neutron fission of 235U was the same as for slow neutron fission, they determined that a pure 235U bomb could have a critical mass of only 6 kg instead of tons, and that the resulting explosion would be tremendous. (The amount actually turned out to be 15 kg, although several times this amount was used in the actual uranium (Little Boy) bomb). In February 1940 they delivered the Frisch–Peierls memorandum. Ironically, they were still officially considered "enemy aliens" at the time.
Glenn Seaborg, Joe Kennedy, Art Wahl and Italian-Jewish refugee Emilio Segrè shortly discovered 239Pu in the decay products of 239U produced by bombarding 238U with neutrons, and determined it to be a fissile material, like 235U.
On June 28, 1941, the Office of Scientific Research and Development was formed in the U.S. to mobilize scientific resources and apply the results of research to national defense. In September, Fermi assembled his first nuclear "pile" or reactor, in an attempt to create a slow neutron-induced chain reaction in uranium, but the experiment failed to achieve criticality, due to lack of proper materials, or not enough of the proper materials which were available.
Producing a fission chain reaction in natural uranium fuel was found to be far from trivial. Early nuclear reactors did not use isotopically enriched uranium, and in consequence they were required to use large quantities of highly purified graphite as neutron moderation materials. Use of ordinary water (as opposed to heavy water) in nuclear reactors requires enriched fuel — the partial separation and relative enrichment of the rare 235U isotope from the far more common 238U isotope. Typically, reactors also require inclusion of extremely chemically pure neutron moderator materials such as deuterium (in heavy water), helium, beryllium, or carbon, the latter usually as graphite. (The high purity for carbon is required because many chemical impurities such as the boron-10 component of natural boron, are very strong neutron absorbers and thus poison the chain reaction.)
Production of such materials at industrial scale had to be solved for nuclear power generation and weapons production to be accomplished. Up to 1940, the total amount of uranium metal produced in the USA was not more than a few grams, and even this was of doubtful purity; of metallic beryllium not more than a few kilograms; and concentrated deuterium oxide (heavy water) not more than a few kilograms. Finally, carbon had never been produced in quantity with anything like the purity required of a moderator.
The problem of producing large amounts of high purity uranium was solved by Frank Spedding using the thermite or "Ames" process. Ames Laboratory was established in 1942 to produce the large amounts of natural (unenriched) uranium metal that would be necessary for the research to come. The critical nuclear chain-reaction success of the Chicago Pile-1 (December 2, 1942) which used unenriched (natural) uranium, like all of the atomic "piles" which produced the plutonium for the atomic bomb, was also due specifically to Szilard's realization that very pure graphite could be used for the moderator of even natural uranium "piles". In wartime Germany, failure to appreciate the qualities of very pure graphite led to reactor designs dependent on heavy water, which in turn was denied the Germans by Allied attacks in Norway, where heavy water was produced. These difficulties prevented the Nazis from building a nuclear reactor capable of criticality during the war.
For more detail on the early development of the first nuclear reactors and nuclear weapons, see Manhattan Project.
References
- ^ http://www.kayelaby.npl.co.uk/atomic_and_nuclear_physics/4_7/4_7_1.html Nuclear Fission and Fusion, and Nuclear Interactions. NLP National Physical Laboratory. 2008. Accessed 2009-06-25.
- ^ L. Bonneau. "Microscopic calculations of potential energy surfaces: fission and fusion properties". Retrieved 2008-07-28.
{{cite web}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Paul K. Kuroda (1956) "On the physical stability of uranium minerals," Journal of Chemical Physics, vol. 25, pages 781–782. Available on-line at: http://nuclearplanet.com/Kuroda%201956.pdf
- ^ Rutherford first published his observations in: Ernest Rutherford (1919) "Collision of alpha particles with light atoms IV. An anomalous effect in nitrogen," Philosophical Magazine, 6th series, vol. 37, pages 581-587. Available on-line at: http://web.lemoyne.edu/~giunta/rutherford.html .
- ^ Cockcroft and Walton first announced their discovery in: J. D. Cockcroft and E. T. S. Walton (30 April 1932) "Letters to the Editor: Disintegration of lithium by swift protons," Nature, vol. 129, page 649. (Available on-line at: http://web.ihep.su/owa/dbserv/hw.part2?s_c=COCKCROFT+1932 .) A more detailed report subsequently appeared in: J. D. Cockcroft and E. T. S. Walton (1 July 1932) "Experiments with high velocity positive ions. II. The disintegration of elements by high velocity protons," Proceedings of the Royal Society of London, series A, vol. 137, no 831, pages 229-242.
- ^ Chadwick announced his initial findings in: James Chadwick (27 Feb. 1932) "Letters to the editor: Possible existence of a neutron," Nature, vol. 129, page 312. (Available on-line at: http://web.mit.edu/22.54/resources/Chadwick.pdf .) Subsequently he communicated his findings in more detail in: (1) Chadwick, J. (1932) "The existence of a neutron," Proceedings of the Royal Society, Series A, vol. 136, pages 692-708 (Available on-line at: http://www.chemteam.info/Chem-History/Chadwick-1932/Chadwick-neutron.html .); and (2) Chadwick, J. (1933) "The Bakerian Lecture: The neutron," Proceedings of the Royal Society, Series A, vol. 142, pages 1-25.
- ^ E. Fermi, E. Amaldi, O. D'Agostino, F. Rasetti, and E. Segrè (1934) "Radioacttività provocata da bombardamento di neutroni III," La Ricerca Scientifica, vol. 5, no. 1, pages 452-453.
- ^ Ida Noddack (1934) "Über das Element 93," Zeitschrift für Angewandte Chemie, vol. 47, no. 37, pages 653-655. Available on-line (in English) at: http://www.chemteam.info/Chem-History/Noddack-1934.html .
- ^ http://www.astr.ua.edu/4000WS/TACKE.html
- ^ Weintraub, Bob. Lise Meitner (1878–1968): Protactinium, Fission, and Meitnerium. Retrieved on June 8, 2009.
- ^ O. Hahn and F. Strassmann. Über den Nachweis und das Verhalten der bei der Bestrahlung des Urans mittels Neutronen entstehenden Erdalkalimetalle ("On the detection and characteristics of the alkaline earth metals formed by irradiation of uranium with neutrons"), Naturwissenschaften Volume 27, Number 1, 11–15 (1939). The authors were identified as being at the Kaiser-Wilhelm-Institut für Chemie, Berlin-Dahlem. Received 22 December 1938.
- ^ Lise Meitner and O. R. Frisch. "Disintegration of Uranium by Neutrons: a New Type of Nuclear Reaction", Nature, Volume 143, Number 3615, 239–240 (11 February 1939). The paper is dated 16 January 1939. Meitner is identified as being at the Physical Institute, Academy of Sciences, Stockholm. Frisch is identified as being at the Institute of Theoretical Physics, University of Copenhagen.
- ^ O. R. Frisch. "Physical Evidence for the Division of Heavy Nuclei under Neutron Bombardment", Nature, Volume 143, Number 3616, 276–276 (18 February 1939). The paper is dated 17 January 1939. [The experiment for this letter to the editor was conducted on 13 January 1939; see Richard Rhodes The Making of the Atomic Bomb. 263 and 268 (Simon and Schuster, 1986).]
- ^ Ruth Lewin Sime. From Exceptional Prominence to Prominent Exception: Lise Meitner at the Kaiser Wilhelm Institute for Chemistry Ergebnisse 24 Forschungsprogramm Geschichte der Kaiser-Wilhelm-Gesellschaft im Nationalsozialismus (2005).
- ^ Ruth Lewin Sime. Lise Meitner: A Life in Physics (University of California, 1997).
- ^ Elisabeth Crawford, Ruth Lewin Sime, and Mark Walker. "A Nobel Tale of Postwar Injustice", Physics Today Volume 50, Issue 9, 26–32 (1997).
- ^ Richard Rhodes. The Making of the Atomic Bomb, 268 (Simon and Schuster, 1986).
- ^ H. L. Anderson, E. T. Booth, J. R. Dunning, E. Fermi, G. N. Glasoe, and F. G. Slack. "The Fission of Uranium", Phys. Rev. Volume 55, Number 5, 511–512 (1939). Institutional citation: Pupin Physics Laboratories, Columbia University, New York, New York. Received 16 February 1939.
- ^ Richard Rhodes. The Making of the Atomic Bomb, 267–270 (Simon and Schuster, 1986).
- DOE Fundamentals Handbook: Nuclear Physics and Reactor Theory Volume 1 (PDF). U.S. Department of Energy. January 1993. Retrieved 2009-02-01.
- DOE Fundamentals Handbook: Nuclear Physics and Reactor Theory Volume 2 (PDF). U.S. Department of Energy. January 1993. Retrieved 2009-02-01.
External links
- The Effects of Nuclear Weapons
- Annotated bibliography for nuclear fission from the Alsos Digital Library
- The Discovery of Nuclear Fission Historical account complete with audio and teacher's guides from the American Institute of Physics History Center
- atomicarchive.com Nuclear Fission Explained
- Nuclear Files.org What is Nuclear Fission?
- Nuclear Fission Animation
