Hyperforin: Difference between revisions
| Line 31: | Line 31: | ||
Hyperforin is also thought to be responsible for the induction of the [[cytochrome P450]] [[enzyme]]s [[CYP3A4]] and [[CYP2C9]] by binding to and activating the [[PXR|pregnane X receptor]] (PXR).<ref name="pmid10852961">{{cite journal |author=Moore LB, Goodwin B, Jones SA, ''et al.'' |title=St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor |journal=Proceedings of the National Academy of Sciences of the United States of America |volume=97 |issue=13 |pages=7500–2 |year=2000 |month=June |pmid=10852961 |pmc=16574 |doi=10.1073/pnas.130155097 |url=}}</ref> |
Hyperforin is also thought to be responsible for the induction of the [[cytochrome P450]] [[enzyme]]s [[CYP3A4]] and [[CYP2C9]] by binding to and activating the [[PXR|pregnane X receptor]] (PXR).<ref name="pmid10852961">{{cite journal |author=Moore LB, Goodwin B, Jones SA, ''et al.'' |title=St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor |journal=Proceedings of the National Academy of Sciences of the United States of America |volume=97 |issue=13 |pages=7500–2 |year=2000 |month=June |pmid=10852961 |pmc=16574 |doi=10.1073/pnas.130155097 |url=}}</ref> |
||
Some [[pharmacokinetic]] data on hyperforin is available for an extract containing 5% hyperforin. Maximal plasma levels ([[Cmax]]) in human volunteers were reached 3.5h after administration of an extract containing 14.8 mg hyperforin. [[Biological half-life]] (t½) and mean residence time were 9h and 12h respectively with an estimated steady state plasma concentration of 100ng/L for 3 doses/d. Linear plasma concentrations were observed within a normal dosage range and no accumulation occured. <ref> Biber A, Fischer H, Römer A, Chatterjee SS. (1998). “Oral bioavailability of hyperforin from hypericum extracts in rats and human volunteers.” ''Pharmacopsychiatry'' 31 Suppl 1:36-43. PMID 9684946 </ref> |
|||
== Antibiotic == |
== Antibiotic == |
||
Revision as of 07:08, 7 October 2010
| |
| Names | |
|---|---|
| IUPAC name
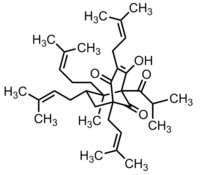
(1R,5S,7R,8S)-4-hydroxy-8-methyl-3,5,7-tris(3-methylbut-2-enyl)-8-(4-methylpent-3-enyl)-1-(2-methylpropanoyl)bicyclo[3.3.1]non-3-ene-2,9-dione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.112.565 |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C35H52O4 | |
| Molar mass | 536.78 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hyperforin is a phytochemical produced by some of the members of the plant genus Hypericum, notably Hypericum perforatum (St John's wort).
Occurrence
Hyperforin has only been found in significant amounts in Hypericum perforatum (St John's wort), [1] where it accumulates in oil glands, pistils, and fruits, probably as a plant defense against herbivory. [2] Other Hypericum species contain low amounts of hyperforin.[3]
Chemistry
The structure of hyperforin was elucidated by a research group from the Shemyakin Institute of Bio-organic Chemistry (USSR Academy of Sciences in Moscow) and published in 1975.[4][5] Hyperforin is a prenylated phloroglucinol derivative. Total synthesis of hyperforin has not yet been accomplished, despite attempts by several research groups.[6]
Hyperforin is unstable in the presence of light and oxygen.[7]
Pharmacology
Hyperforin is believed to be the primary active constituent responsible for the antidepressant and anxiolytic properties of the extracts of St. John's wort.[8] It acts as a reuptake inhibitor of monoamines, including serotonin, norepinephrine, dopamine, and of GABA and glutamate, with IC50 values of 0.05-0.10 mcg/ml for all compounds, with the exception of glutamate, which is in the 0.5 mcg/ml range.[9] It appears to exert these effects by activating the transient receptor potential ion channel TRPC6.[10] Activation of TRPC6 induces the entry of sodium and calcium into the cell which causes inhibition of monoamine reuptake.[10]
Hyperforin is also thought to be responsible for the induction of the cytochrome P450 enzymes CYP3A4 and CYP2C9 by binding to and activating the pregnane X receptor (PXR).[11]
Some pharmacokinetic data on hyperforin is available for an extract containing 5% hyperforin. Maximal plasma levels (Cmax) in human volunteers were reached 3.5h after administration of an extract containing 14.8 mg hyperforin. Biological half-life (t½) and mean residence time were 9h and 12h respectively with an estimated steady state plasma concentration of 100ng/L for 3 doses/d. Linear plasma concentrations were observed within a normal dosage range and no accumulation occured. [12]
Antibiotic
Hyperforin has antibiotic properties and is active against methicillin-resistant strains of Staphylococcus aureus (MRSA) with a minimal inhibitory concentration (MIC) value of 1.0 μg/ml,[13] as well as against other gram-positive bacteria.[14]
See also
Further reading
- Beerhues L (2006). "Molecule of Interest: Hyperforin". Phytochemistry. 67 (20): 2201–7. doi:10.1016/j.phytochem.2006.08.017. PMID 16973193.
{{cite journal}}: Unknown parameter|month=ignored (help)
References
- ^ Umek A, Kreft S, Kartnig T, Heydel B (1999). "Quantitative phytochemical analyses of six hypericum species growing in slovenia". Planta medica. 65 (4): 388–90. doi:10.1055/s-2006-960798. PMID 17260265.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Beerhues L. (2006). “Hyperforin.” Phytochemistry 67 (20): 2201-7. PMID 16973193
- ^ Smelcerovic A, Spiteller M (2006). "Phytochemical analysis of nine Hypericum L. species from Serbia and the F.Y.R. Macedonia". Die Pharmazie. 61 (3): 251–2. PMID 16599273.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Bystrov NS, Gupta ShR, Dobrynin VN, Kolosov MN, Chernov BK (1976). "[Structure of the antibiotic hyperforin]". Doklady Akademii Nauk SSSR (in Russian). 226 (1): 88–90. PMID 1248360.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bystrov NS, Chernov BK, Dobrynin VN, Kolosov MN (1975). "[The structure of hyperforin]". Tetrahedron Letters. 16 (32): 2791–2794. doi:10.1016/S0040-4039(00)75241-5.
{{cite journal}}: Cite has empty unknown parameter:|month=(help)CS1 maint: multiple names: authors list (link) - ^ Nicolaou KC, Carenzi GE, Jeso V (2005). "Construction of highly functionalized medium-sized rings: synthesis of hyperforin and perforatumone model systems". Angewandte Chemie (International ed. In English). 44 (25): 3895–9. doi:10.1002/anie.200500776. PMID 15892032.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Liu F, Pan C, Drumm P, Ang CY (2005). "Liquid chromatography-mass spectrometry studies of St. John's wort methanol extraction: active constituents and their transformation". Journal of pharmaceutical and biomedical analysis. 37 (2): 303–12. doi:10.1016/j.jpba.2004.10.034. PMID 15708671.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Newall, Carol A.; Joanne Barnes; Anderson, Linda R. (2002). Herbal medicines: a guide for healthcare professionals. London: Pharmaceutical Press. ISBN 0-85369-474-5.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Chatterjee SS, Bhattacharya SK, Wonnemann M, Singer A, Müller WE (1998). "Hyperforin as a possible antidepressant component of hypericum extracts". Life Sci. 63 (6): 499–510. doi:10.1016/S0024-3205(98)00299-9. PMID 9718074.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Leuner K, Kazanski V, Müller M; et al. (2007). "Hyperforin--a key constituent of St. John's wort specifically activates TRPC6 channels". The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 21 (14): 4101–11. doi:10.1096/fj.07-8110com. PMID 17666455.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Moore LB, Goodwin B, Jones SA; et al. (2000). "St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor". Proceedings of the National Academy of Sciences of the United States of America. 97 (13): 7500–2. doi:10.1073/pnas.130155097. PMC 16574. PMID 10852961.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Biber A, Fischer H, Römer A, Chatterjee SS. (1998). “Oral bioavailability of hyperforin from hypericum extracts in rats and human volunteers.” Pharmacopsychiatry 31 Suppl 1:36-43. PMID 9684946
- ^ Reichling J, Weseler A, Saller R (2001). "A current review of the antimicrobial activity of Hypericum perforatum L". Pharmacopsychiatry. 34 Suppl 1: S116–8. doi:10.1055/s-2001-15514. PMID 11518059.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Schempp CM, Pelz K, Wittmer A, Schöpf E, Simon JC (1999). "Antibacterial activity of hyperforin from St John's wort, against multiresistant Staphylococcus aureus and gram-positive bacteria". Lancet. 353 (9170): 2129. doi:10.1016/S0140-6736(99)00214-7. PMID 10382704.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
