Nitrate: Difference between revisions
Edited caption |
No edit summary |
||
| Line 1: | Line 1: | ||
[[File:Nitrate-ion-resonance-hybrid-2D.png|thumb|150px|The nitrate ion. The net charge of the whole ion is 1<sup>−</sup>.]] |
[[File:Nitrate-ion-resonance-hybrid-2D.png|thumb|150px|The nitrate ion. The net charge of the whole ion is 1<sup>−</sup>.]] |
||
The '''nitrate ion''' is a [[polyatomic]] [[ion]] with the [[molecular formula]] NO{{su|b=3|p=−}} and a [[molecular mass]] of 62.0049 g/mol. It is the [[conjugate acid|conjugate base]] of [[nitric acid]], consisting of one central [[nitrogen]] [[atom]] surrounded by three identical oxygen atoms in a [[trigonal planar]] arrangement. The nitrate ion carries a [[formal charge]] of negative one, where each oxygen carries a −{{frac|2|3}} charge whereas the nitrogen carries a |
The '''nitrate ion''' is a [[polyatomic]] [[ion]] with the [[molecular formula]] NO{{su|b=3|p=−}} and a [[molecular mass]] of 62.0049 g/mol. It is the [[conjugate acid|conjugate base]] of [[nitric acid]], consisting of one central [[nitrogen]] [[atom]] surrounded by three identical oxygen atoms in a [[trigonal planar]] arrangement. The nitrate ion carries a [[formal charge]] of negative one, where each oxygen carries a −{{frac|2|3}} charge whereas the nitrogen carries a -1 charge, and is commonly used as an example of [[Resonance (chemistry)|resonance]]. Like the [[isoelectronic]] [[carbonate]] ion, the nitrate ion can be represented by resonance structures: |
||
<div style="text-align: center">[[Image:Nitrate-ion-resonance-2D.png|400px|Canonical resonance structures for the nitrate ion]]</div> |
<div style="text-align: center">[[Image:Nitrate-ion-resonance-2D.png|400px|Canonical resonance structures for the nitrate ion]]</div> |
||
Revision as of 01:32, 11 October 2010
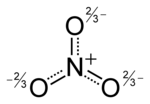
The nitrate ion is a polyatomic ion with the molecular formula NO−
3 and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identical oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of negative one, where each oxygen carries a −2⁄3 charge whereas the nitrogen carries a -1 charge, and is commonly used as an example of resonance. Like the isoelectronic carbonate ion, the nitrate ion can be represented by resonance structures:
Almost all inorganic nitrate salts are soluble in water at standard temperature and pressure.
In organic chemistry a nitrate (not to be confused with nitro) is a functional group with general chemical formula RONO2 where R stands for any organic residue. They are the esters of nitric acid and alcohols formed by nitroxylation. Examples are methyl nitrate formed by reaction of methanol and nitric acid,[1] the nitrate of tartaric acid,[2] and the inappropriately named nitroglycerin.
Related materials
Nitrate should not be confused with nitrite (NO−
2), the salts of nitrous acid. Organic compounds containing the nitro functional group (which has the same formula and structure as the nitrate ion save that one of the O− atoms is replaced by the group) are known as nitro compounds.
Human health effects
Nitrate toxicosis in humans occurs through enterohepatic metabolism of nitrate to ammonia, with nitrite being an intermediate[3]. Nitrites oxidize the iron atoms in hemoglobin from ferrous iron (2+) to ferric iron (3+), rendering it unable to carry oxygen[4]. This process can lead to generalized lack of oxygen in organ tissue and a dangerous condition called methemoglobinemia. Methemoglobinemia can be treated with methylene blue, which reduces ferric iron (3+) in affected blood cells back to ferrous iron (2+).
Infants in particular are especially vulnerable to methemoglobinemia due to nitrate metabolizing triglycerides present at higher concentrations than at other stages of development. Methemoglobinemia in infants is known as blue baby syndrome. There are now significant scientific doubts as to whether there is a causal link between nitrates in drinking water and 'blue baby syndrome'.[5][6] Blue baby syndrome is now thought to be the product of a number of factors, which can include any factor which causes gastric upset, such as diarrhoeal infection, protein intolerance, heavy metal toxicity etc., with nitrates playing a minor role. Nitrates, if a factor in a specific case, would most often be ingested by infants in high nitrate drinking water. However, nitrate exposure may also occur if eating, for instance, vegetables containing high levels of nitrate. Lettuce may contain elevated nitrate under growth conditions such as reduced sunlight, undersupply of the essential micronutrients molybdenum (Mo) and iron (Fe), or high concentrations of nitrate due to reduced assimilation of nitrate in the plant. High levels of nitrate fertilization also contribute to elevated levels of nitrate in the harvested plant .[7]
Some adults can be more susceptible to the effects of nitrate than others. The methemoglobin reductase enzyme may be under-produced or absent in certain people that have an inherited mutation[8]. Such individuals cannot break down methemoglobin as rapidly as those that do have the enzyme, leading to increased circulating levels of methemoglobin (the implication being that their blood is not as oxygen-rich). Those with insufficient stomach acid[9] (including some vegetarians and vegans) may also be at risk. It is the increased consumption of green, leafy vegetables that typically accompany these types of diets may lead to increased nitrate intake. A wide variety of medical conditions, including food allergies, asthma[10], hepatitis, and gallstones may be linked with low stomach acid; these individuals may also be highly sensitive to the effects of nitrate.
Marine toxicity

In freshwater or estuarine systems close to land, nitrate can reach high levels that can potentially cause the death of fish. While nitrate is much less toxic than ammonia or nitrite,[11] levels over 30 ppm of nitrate can inhibit growth, impair the immune system and cause stress in some aquatic species.[12] However, in light of inherent problems with past protocols on acute nitrate toxicity experiments, the extent of nitrate toxicity has been the subject of recent debate.[13]
In most cases of excess nitrate concentrations in aquatic systems, the primary source is surface runoff from agricultural or landscaped areas that have received excess nitrate fertilizer. These levels of nitrate can also lead to algae blooms, and when nutrients become limiting (such as potassium, phosphate or nitrate) then eutrophication can occur. As well as leading to water anoxia and dead zones, these blooms may cause other changes to ecosystem function, favouring some groups of organisms over others. As a consequence, as nitrate forms a component of total dissolved solids, they are widely used as an indicator of water quality.
Nitrate also is a by-product of septic systems. To be specific, it is a naturally occurring chemical that is left after the breakdown or decomposition of animal or human waste. Water quality may also be affected through ground water resources that have a high number of septic systems in a watershed. Septics leach down into ground water resources or aquifers and supply nearby bodies of water. Lakes that rely on ground water are often affected by nitrification through this process.
See also
- Ammonium
- f-ratio
- Nitrification
- Peroxynitrate
- Nitratine
- Nitrate overview:
External links
References
- ^ Alvin P. Black and Frank H. Babers. "Methyl nitrate". Organic Syntheses; Collected Volumes, vol. 2, p. 412.
- ^ Snyder, H. R.; Handrick, R. G.; Brooks, L. A. (1943). "Imidazole". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 3, p. 471. - ^ "Nitrate and Nitrite Poisoning: Introduction". The Merck Veterinary Manual. Retrieved 2008-12-27.
- ^ Kim-shapiro, D.B.; Gladwin, M.T.; Patel, R.P.; Hogg, N. (2005). "… between nitrite and hemoglobin: the role of nitrite in hemoglobin-mediated hypoxic vasodilation". Journal of Inorganic Biochemistry. 99 (1): 237–246. doi:10.1016/j.jinorgbio.2004.10.034. PMID 15598504.
- ^ T.M Addiscott & N Benjamin 2004 Nitrate and human health
- ^ A A Avery Infant Methemoglobemia - reexamining the role of drinking water nitrates
- ^ Marschner H 1999 Mineral nutrition of higher plants. Academic Press, London. 889
- ^ Washington State Department of Health. "Nitrate in Drinking Water." Accessed: September 2, 2009. Available from http://www.doh.wa.gov/ehp/dw/Programs/nitrate.htm
- ^ Washington State Department of Health. "Nitrate in Drinking Water." Accessed: September 2, 2009. Available from http://www.doh.wa.gov/ehp/dw/Programs/nitrate.htm
- ^ WebMD. "GERD and Asthma." Accessed: September 2, 2009. Available from http://www.webmd.com/asthma/guide/heartburn-asthma
- ^ Romano, N.; Zeng, C. (2007). "Acute toxicity of sodium nitrate, potassium nitrate and potassium chloride and their effects on the hemolymph composition and gill structure of early juvenile blue swimmer crabs (Portunus pelagicus, Linneaus 1758) (Decapoda, Brachyura, Portunidae)." Environmental Toxicology and Chemistry 26: 1955–1962.
- ^ Nitrates in the Aquarium
- ^ Romano N., Zeng, C. (2007). "Effects of potassium on nitrate mediated changes to osmoregulation in marine crabs". Aquatic Toxicology. 85 (3): 202–208. doi:10.1016/j.aquatox.2007.09.004. PMID 17942166.
{{cite journal}}: CS1 maint: multiple names: authors list (link)

