Bisabolol: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Chembox |
{{Chembox |
||
| Name = ''α''-(-)-Bisabolol |
|||
| ImageFile = Bisabolol-Line-Structure.svg |
| ImageFile = Bisabolol-Line-Structure.svg |
||
| ImageSize = |
| ImageSize = 244 |
||
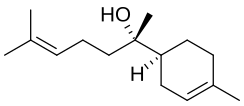
| ImageName = Stereo structural formula of |
| ImageName = Stereo structural formula of (2R)-6-methyl,(1S)-4-methyl-bisabolol |
||
| PIN = 6-Methyl-2-(4-methylcyclohex-3-enyl)hept-5-en-2-ol |
| PIN = 6-Methyl-2-(4-methylcyclohex-3-enyl)hept-5-en-2-ol |
||
| SystematicName = 6-Methyl-2-(4-methylcyclohex-3-en-1-yl)hept-5-en-2-ol |
| SystematicName = 6-Methyl-2-(4-methylcyclohex-3-en-1-yl)hept-5-en-2-ol |
||
| OtherNames = |
| OtherNames = Anymol<br /> |
||
α-Bisabolol<br /> |
|||
α-Bisabolol<br /> |
|||
Dragosantol<br /> |
|||
Levomenol |
Levomenol |
||
| Section1 = {{Chembox Identifiers |
| Section1 = {{Chembox Identifiers |
||
| CASNo = 515-69-5 |
| CASNo = 515-69-5 |
||
| CASNo_Ref = {{ |
| CASNo_Ref = {{cascite|correct|CAS}} |
||
| PubChem = 10586 |
| PubChem = 10586 |
||
| PubChem_Ref = {{Pubchemcite|correct|PubChem}} |
| PubChem_Ref = {{Pubchemcite|correct|PubChem}} |
||
| PubChem1 = 12300144 |
| PubChem1 = 12300144 |
||
| PubChem1_Comment = (1''S'') |
| PubChem1_Comment = (1''S'')-4-methyl |
||
| PubChem1_Ref = {{Pubchemcite|correct|PubChem}} |
| PubChem1_Ref = {{Pubchemcite|correct|PubChem}} |
||
| PubChem2 = 6097621 |
| PubChem2 = 6097621 |
||
| PubChem2_Comment = (2''S'') |
| PubChem2_Comment = (2''S'')-6-methyl |
||
| PubChem2_Ref = {{Pubchemcite|correct|PubChem}} |
| PubChem2_Ref = {{Pubchemcite|correct|PubChem}} |
||
| PubChem3 = 1549992 |
|||
| PubChem3_Comment = (2''R'')-6-methyl,(1''R'')-4-methyl |
|||
| PubChem3_Ref = {{Pubchemcite|correct|PubChem}} |
|||
| PubChem4 = 1616126 |
|||
| PubChem4_Comment = (2''R'')-6-methyl,(1''S'')-4-methyl |
|||
| PubChem4_Ref = {{Pubchemcite|correct|PubChem}} |
|||
| ChemSpiderID = 10141 |
| ChemSpiderID = 10141 |
||
| ChemSpiderID_Ref = {{Chemspidercite|correct|ChemSpider}} |
| ChemSpiderID_Ref = {{Chemspidercite|correct|ChemSpider}} |
||
| ChemSpiderID1 = 4807789 |
|||
| ChemSpiderID1_Comment = (2''S'')-6-methyl |
|||
| ChemSpiderID1_Ref = {{Chemspidercite|correct|ChemSpider}} |
|||
| ChemSpiderID2 = 1266723 |
|||
| ChemSpiderID2_Comment = (2''R'')-6-methyl,(1''R'')-4-methyl |
|||
| ChemSpiderID2_Ref = {{Chemspidercite|correct|ChemSpider}} |
|||
| ChemSpiderID3 = 1299417 |
|||
| ChemSpiderID3_Comment = (2''R'')-6-methyl,(1''S'')-4-methyl |
|||
| ChemSpiderID3_Ref = {{Chemspidercite|correct|ChemSpider}} |
|||
| UNII = 24WE03BX2T |
| UNII = 24WE03BX2T |
||
| UNII_Ref = {{ |
| UNII_Ref = {{fdacite|correct|FDA}} |
||
| EINECS = 208-205-9 |
| EINECS = 208-205-9 |
||
| MeSHName = Bisabolol |
| MeSHName = Bisabolol |
||
| ChEBI = 585092 |
|||
| RTECS = MJ9685000 |
| RTECS = MJ9685000 |
||
| SMILES = CC(C)=CCCC(C)(O)C1CCC(C)=CC1 |
| SMILES = CC(C)=CCCC(C)(O)C1CCC(C)=CC1 |
||
| SMILES1 = O[C](C)(CC\C=C(/C)C)[CH]1C/C=C(/C)CC1 |
| SMILES1 = O[C](C)(CC\C=C(/C)C)[CH]1C/C=C(/C)CC1 |
||
| |
| StdInChI = 1S/C15H26O/c1-12(2)6-5-11-15(4,16)14-9-7-13(3)8-10-14/h6-7,14,16H,5,8-11H2,1-4H3 |
||
| |
| InChI = 1/C15H26O/c1-12(2)6-5-11-15(4,16)14-9-7-13(3)8-10-14/h6-7,14,16H,5,8-11H2,1-4H3/t14-,15+/m1/s1 |
||
| |
| StdInChIKey = RGZSQWQPBWRIAQ-UHFFFAOYSA-N |
||
| InChIKey1 = RGZSQWQPBWRIAQ-CABCVRREBV |
| InChIKey1 = RGZSQWQPBWRIAQ-CABCVRREBV |
||
| Beilstein = 5733954}} |
| Beilstein = 5733954}} |
||
| Line 38: | Line 56: | ||
| O = 1 |
| O = 1 |
||
| ExactMass = 222.198365454 g mol<sup>-1</sup> |
| ExactMass = 222.198365454 g mol<sup>-1</sup> |
||
| Density = 0.92 g |
| Density = 0.92 g cm<sup>-3</sup> |
||
| |
| BoilingPtC = 153 |
||
| |
| Boiling_notes = at 12 mmHg}} |
||
}} |
}} |
||
'''Bisabolol''', or more formally α-(-)-bisabolol or also known as levomenol <ref>[http://www.omikron-online.de/naturhaus/angebote/info/bisabolo.htm Rohstoff-Lexikon Bisabolol<!-- Bot generated title -->]</ref>, is a natural monocyclic [[sesquiterpene]] alcohol. It is a colorless viscous oil that is the primary constituent of the [[essential oil]] from [[German chamomile]] (''Matricaria recutita'') and ''Myoporum grassifolium''<ref>[http://www.omikron-online.de/naturhaus/angebote/info/bisab.htm Bisabolol (in english)<!-- Bot generated title -->]</ref>. It is almost insoluble in water and [[glycerin]], but well soluble in [[ethanol]]. The [[enantiomer]], α-(+)-bisabolol, is also found naturally but is rare. Synthetic bisabolol is usually a [[racemic]] mixture of the two, α-(±)-bisabolol. |
'''Bisabolol''', or more formally α-(-)-bisabolol or also known as levomenol <ref>[http://www.omikron-online.de/naturhaus/angebote/info/bisabolo.htm Rohstoff-Lexikon Bisabolol<!-- Bot generated title -->]</ref>, is a natural monocyclic [[sesquiterpene]] alcohol. It is a colorless viscous oil that is the primary constituent of the [[essential oil]] from [[German chamomile]] (''Matricaria recutita'') and ''Myoporum grassifolium''<ref>[http://www.omikron-online.de/naturhaus/angebote/info/bisab.htm Bisabolol (in english)<!-- Bot generated title -->]</ref>. It is almost insoluble in water and [[glycerin]], but well soluble in [[ethanol]]. The [[enantiomer]], α-(+)-bisabolol, is also found naturally but is rare. Synthetic bisabolol is usually a [[racemic]] mixture of the two, α-(±)-bisabolol. |
||
Revision as of 23:46, 11 November 2010
| |
| Names | |
|---|---|
| Preferred IUPAC name
6-Methyl-2-(4-methylcyclohex-3-enyl)hept-5-en-2-ol | |
| Systematic IUPAC name
6-Methyl-2-(4-methylcyclohex-3-en-1-yl)hept-5-en-2-ol | |
| Other names
Anymol
α-Bisabolol | |
| Identifiers | |
3D model (JSmol)
|
|
| 5733954 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.041.279 |
| EC Number |
|
| MeSH | Bisabolol |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H26O | |
| Molar mass | 222.372 g·mol−1 |
| Density | 0.92 g cm-3 |
| Boiling point | 153 °C (307 °F; 426 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bisabolol, or more formally α-(-)-bisabolol or also known as levomenol [1], is a natural monocyclic sesquiterpene alcohol. It is a colorless viscous oil that is the primary constituent of the essential oil from German chamomile (Matricaria recutita) and Myoporum grassifolium[2]. It is almost insoluble in water and glycerin, but well soluble in ethanol. The enantiomer, α-(+)-bisabolol, is also found naturally but is rare. Synthetic bisabolol is usually a racemic mixture of the two, α-(±)-bisabolol.
Bisabolol has a weak sweet floral aroma and is used in various fragrances. It has also been used for hundreds of years in cosmetics because of its perceived skin healing properties. Bisabolol is known to have anti-irritant, anti-inflammatory and anti-microbial properties. Bisabolol is also demonstrated to enhance the percutaneous absorption of certain molecules.[3]
A structurally related compound known as β-bisabolol (CAS registry number [15352-77-9]) differs only in the position of the tertiary alcohol functional group.

References
- ^ Rohstoff-Lexikon Bisabolol
- ^ Bisabolol (in english)
- ^ J Am Oil Chem Soc (2010) 87;1-7.
