Pearlite: Difference between revisions
No edit summary |
ce |
||
| Line 1: | Line 1: | ||
{{for|the [[amorphous]] volcanic glass|perlite}} |
{{for|the [[amorphous]] volcanic glass|perlite}} |
||
{{Steels}} |
{{Steels}} |
||
[[Image:pearlite.jpg|thumb |
[[Image:pearlite.jpg|thumb|[[Scanning Electron Microscope|SEM]] micrograph of etched pearlite, 2000X.]] |
||
[[Image:Phase diag iron carbon.PNG|thumb |
[[Image:Phase diag iron carbon.PNG|thumb|Pearlite occurs at the [[eutectoid]] of the iron-carbon phase diagram (near the lower left).]] |
||
'''Pearlite''' is a [[Phase (matter)|two-phased]], [[Lamellar structure|lamellar]] (or layered) structure composed of alternating layers of |
|||
alpha-[[Ferrite (iron)|ferrite]] (88 wt%) and [[cementite]] (12%) that occurs in some [[steel]]s and [[cast iron]]s. During slow cooling pearlite forms by a [[eutectoid]] reaction as [[austenite]] is below 727 |
'''Pearlite''' is a [[Phase (matter)|two-phased]], [[Lamellar structure|lamellar]] (or layered) structure composed of alternating layers of alpha-[[Ferrite (iron)|ferrite]] (88 wt%) and [[cementite]] (12%) that occurs in some [[steel]]s and [[cast iron]]s. During slow cooling pearlite forms by a [[eutectoid]] reaction as [[austenite]] is below {{convert|727|C|abbr=on}} (the eutectoid temperature). Pearlite is a common microstructure occurring in many grades of steels. |
||
The eutectoid composition of austenite is approximately 0.8% [[carbon]]; steel with less carbon content will contain a corresponding proportion of relatively pure ferrite crystallites that do not participate in the eutectoid reaction and cannot transform into pearlite. Likewise steels with higher carbon contents will form cementite before reaching the eutectoid point. The proportion of ferrite and cementite forming above the eutectoid point can be calculated from the iron/iron—carbide equilibrium phase diagram using the [[lever rule]]. |
The eutectoid composition of austenite is approximately 0.8% [[carbon]]; steel with less carbon content will contain a corresponding proportion of relatively pure ferrite crystallites that do not participate in the eutectoid reaction and cannot transform into pearlite. Likewise steels with higher carbon contents will form cementite before reaching the eutectoid point. The proportion of ferrite and cementite forming above the eutectoid point can be calculated from the iron/iron—carbide equilibrium phase diagram using the [[lever rule]]. |
||
Pearlite was first identified by [[Henry Clifton Sorby]] and initially named sorbite, however the similarity of microstructure to [[mother of pearl|nacre]] and especially the optical effect caused by the scale of the structure made the alternative name more popular. |
Pearlite was first identified by [[Henry Clifton Sorby]] and initially named sorbite, however the similarity of microstructure to [[mother of pearl|nacre]] and especially the optical effect caused by the scale of the structure made the alternative name more popular. |
||
Pearlite is a common microstructure occurring in many grades of steels. |
|||
[[Bainite]] is a similar structure with lamellae much smaller than the [[wavelength]] of [[visible light]] and thus lacks this pearlescent appearance. It is prepared by more rapid cooling. Unlike pearlite, whose formation involves the diffusion of all atoms, bainite grows by a displacive transformation mechanism. |
[[Bainite]] is a similar structure with lamellae much smaller than the [[wavelength]] of [[visible light]] and thus lacks this pearlescent appearance. It is prepared by more rapid cooling. Unlike pearlite, whose formation involves the diffusion of all atoms, bainite grows by a displacive transformation mechanism. |
||
==Eutectoid steel== |
|||
Pearlite is sometimes also called as [[Eutectoid steel]] as it has 0.8% carbon and is the result of the reversible eutectoid reaction of ferrite and cementite. |
|||
| ⚫ | Eutectoid steel is a steel made up completely of pearlite. It has very high strength, hardness and toughness. It has excellent wear resistance because of a strong lamellar network of ferrite and cementite. Examples of applications include [[cutting tool]]s, high strength [[wire]]s, [[knive]]s, [[chisel]]s, and [[nails]]. |
||
==Properties== |
|||
| ⚫ | |||
==Application== |
|||
Because of its good strength and [[hardness]], it is used for making cutting tool materials, High strength wires, [[Knives]], [[chisels]], punch [[nails]] etc. |
|||
==References== |
==References== |
||
{{No footnotes|date=September 2009}} |
{{No footnotes|date=September 2009}} |
||
[http://www.msm.cam.ac.uk/phase-trans/2005/pearlite.html Comprehensive information on pearlite] |
*[http://www.msm.cam.ac.uk/phase-trans/2005/pearlite.html Comprehensive information on pearlite] |
||
*'''Introduction to Physical metallurgy''' by Sidney |
*'''Introduction to Physical metallurgy''' by Sidney H. Avner, second edition, McGraw hill publications. |
||
[[Category:Metallurgy]] |
[[Category:Metallurgy]] |
||
Revision as of 20:51, 15 November 2010
| Steels |
|---|
 |
| Phases |
| Microstructures |
| Classes |
| Other iron-based materials |

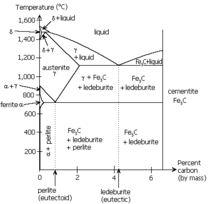
Pearlite is a two-phased, lamellar (or layered) structure composed of alternating layers of alpha-ferrite (88 wt%) and cementite (12%) that occurs in some steels and cast irons. During slow cooling pearlite forms by a eutectoid reaction as austenite is below 727 °C (1,341 °F) (the eutectoid temperature). Pearlite is a common microstructure occurring in many grades of steels.
The eutectoid composition of austenite is approximately 0.8% carbon; steel with less carbon content will contain a corresponding proportion of relatively pure ferrite crystallites that do not participate in the eutectoid reaction and cannot transform into pearlite. Likewise steels with higher carbon contents will form cementite before reaching the eutectoid point. The proportion of ferrite and cementite forming above the eutectoid point can be calculated from the iron/iron—carbide equilibrium phase diagram using the lever rule.
Pearlite was first identified by Henry Clifton Sorby and initially named sorbite, however the similarity of microstructure to nacre and especially the optical effect caused by the scale of the structure made the alternative name more popular.
Bainite is a similar structure with lamellae much smaller than the wavelength of visible light and thus lacks this pearlescent appearance. It is prepared by more rapid cooling. Unlike pearlite, whose formation involves the diffusion of all atoms, bainite grows by a displacive transformation mechanism.
Eutectoid steel
Eutectoid steel is a steel made up completely of pearlite. It has very high strength, hardness and toughness. It has excellent wear resistance because of a strong lamellar network of ferrite and cementite. Examples of applications include cutting tools, high strength wires, knives, chisels, and nails.
References
This article includes a list of references, related reading, or external links, but its sources remain unclear because it lacks inline citations. (September 2009) |
- Comprehensive information on pearlite
- Introduction to Physical metallurgy by Sidney H. Avner, second edition, McGraw hill publications.
