Arsenic triiodide: Difference between revisions
No edit summary |
No edit summary |
||
| Line 57: | Line 57: | ||
{{Arsenic compounds}} |
{{Arsenic compounds}} |
||
| ⚫ | |||
[[Category:Inorganic compound stubs]] |
[[Category:Inorganic compound stubs]] |
||
[[Category:Iodides]] |
[[Category:Iodides]] |
||
| ⚫ | |||
{{inorganic-compound-stub}} |
{{inorganic-compound-stub}} |
||
Revision as of 07:21, 24 November 2010
| |
| Names | |
|---|---|
| Preferred IUPAC name
Arsenic triiodide | |
| Systematic IUPAC name
Triiodoarsane | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.153 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| AsI3 | |
| Molar mass | 455.635 g/mol |
| Appearance | orange-red crystalline solid |
| Density | 4.69 g/cm3 |
| Melting point | 146 °C |
| Boiling point | 403 °C |
| 6 g/100 mL | |
| Solubility | soluble in alcohol, ether, CS2 |
Refractive index (nD)
|
2.23 |
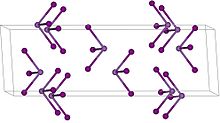
| Structure | |
| Rhombohedral, hR24, SpaceGroup = R-3, No. 148 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Arsenic triiodide is the inorganic compound with the formula AsI3. It is a dark red solid that readily sublimes. It is a pyramidial molecule that is useful for preparing organoarsenic compounds.
Preparation
It is prepared by a reaction of arsenic trichloride and potassium iodide:[2]
- AsCl3 + 3KI → AsI3 + 3 KCl
Reactions
Hydrolysis occurs only slowly in water forming arsenic trioxide and hydroiodic acid. The reaction proceeds via formation of arsenous acid which exists in equilibrium with hydroiodic acid. The aqueous solution is highly acidic, pH of 0.1N solution is 1.1. It decomposes to arsenic trioxide, elemental arsenic and iodine when heated in air at 200 °C. The decomposition, however, commences at 100 °C and occurs with the liberation of iodine.
Former uses
Under the name of Donovan's solution, it was once recommended to treat rheumatism, arthritis, malaria, trypanosome infections, tuberculosis, and diabetes.[1]
References
- ^ a b Shakhashiri BZ, "Chemical of the Week: Arsenic", University of Wisconsin–Madison Chemistry Dept.
- ^ John C. Bailar, Jr. "Arsenic Triiodide" Inorganic Syntheses 1939, volume 1, pp. 103–104, 2007. doi:10.1002/9780470132326.ch36
