Methylsulfonylmethane: Difference between revisions
No edit summary |
|||
| Line 64: | Line 64: | ||
==Structure and chemical properties== |
==Structure and chemical properties== |
||
MSN is structurally related to [[dimethyl sulfoxide]] (DMSO), but the behavior of these two is different. DMSO is a highly polar solvent and an excellent ligand, with water-like dissolving properties whereas MSM is less polar and less reactive. MSM is also a [[metabolite]] of DMSO. |
|||
==Use as a solvent== |
==Use as a solvent== |
||
Revision as of 18:19, 21 January 2011
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
dimethyl sulfone
| |||
| Preferred IUPAC name
methanesulfonylmethane | |||
| Other names
methyl sulfone
methylsulfonylmethane sulfonylbismethane DMSO2 | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.605 | ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H6O2S | |||
| Molar mass | 94.13 g/mol | ||
| Appearance | White crystalline solid | ||
| Density | 1.45 g/cm3 | ||
| Melting point | 109 °C (228 °F; 382 K) | ||
| Boiling point | 238 °C (460 °F; 511 K) | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 143 °C | ||
| Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
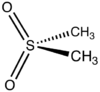
Methylsulfonylmethane (MSN) is an organosulfur compound with the formula (CH3)2SO2. It is also known by several other names including DMSO2, methyl sulfone, and dimethyl sulfone. [1] This colorless solid features the sulfonyl functional group and is considered relatively inert chemically. It occurs naturally in some primitive plants and is present in small amounts in many foods and beverages and it is marketed as a dietary supplement, although its benefits are disputed.
Structure and chemical properties
MSN is structurally related to dimethyl sulfoxide (DMSO), but the behavior of these two is different. DMSO is a highly polar solvent and an excellent ligand, with water-like dissolving properties whereas MSM is less polar and less reactive. MSM is also a metabolite of DMSO.
Use as a solvent
Because of its polarity and thermal stability, MSM is used industrially as a high-temperature solvent for both inorganic and organic substances. It is used as a medium in organic synthesis. For example, displacement of aryl chlorides by potassium fluoride can be usefully conducted in molten MSM.[2] With a pKa of 31, it can be deprotonated with sodium amide, and the conjugate base is an effective nucleophile.
Effects on health
MSM is promoted as a natural source of sulfur by the supplement and health food industry. There is no Dietary Reference Intake (DRI) or Daily Value established for sulfur. MSM is sold as a dietary supplement that is marketed with a variety of claims, and is commonly used (often in combination with glucosamine and/or chondroitin) for helping to treat or prevent osteoarthritis. The biochemical effects of supplemental methylsulfonylmethane are poorly understood. Some researchers have suggested that MSM has anti-inflammatory effects.[3][4] Any health effects of dimethyl sulfoxide (DMSO) may be mediated, at least in part, by MSM.[5][6] Stanley W. Jacob, M.D., of the Oregon Health and Science University, reports using MSM to treat over 18,000 patients with a variety of ailments.[7] According to one review, "The benefits claimed far exceed the number of scientific studies. It is hard to build a strong case for its use other than for treating arthritis problems."[8]
Evidence from clinical trials
This section possibly contains synthesis of material that does not verifiably mention or relate to the main topic. (August 2010) |
Clinical evidence for the usefulness of MSM consists of published studies on both animals and humans. These studies of MSM have suggested some benefits, particularly for treatment of osteoarthritis. Further studies would be needed to test the usefulness of the chemical as a medical therapy.
- Osteoarthritis: After several reports that MSM helped arthritis in animal models, a double-blind, placebo-controlled study suggested that 1500 mg per day MSM (alone or in combination with glucosamine sulfate) was helpful in relieving symptoms of knee osteoarthritis[9] The results of the Usha clinical trial are suspect.[by whom?] It was conducted in India by researchers with very little prior experience in clinical trials.[improper synthesis?] Tests are described in Methods but no data presented, and for other tests statements in Results and Discussion are not supported by the data shown.[improper synthesis?] Discussion statements are not supported by the references cited.[improper synthesis?] Placebo group results did not significantly change from baseline, whereas in osteoarthritis trials there is typically a 30-40% improvement in the placebo group.[improper synthesis?] Kim et al. conducted a double-blind clinical trial of MSM for treatment of patients with osteoarthritis of the knee. Twenty-five patients took 6000 mg/day MSM and 25 patients took a placebo for 12 weeks. Ten patients did not complete the study, and intention to treat analysis was performed. Patients who took MSM had significantly reduced pain and improved physical functioning, without major adverse events. No evidence of a more general anti-inflammatory effect was found, as there were no significant changes in two measures of systemic inflammation: C-reactive protein level and erythrocyte sedimentation rate. The authors cautioned that this short pilot study did not address the long-term safety and usefulness of MSM, but suggested that physicians should consider its use for certain osteoarthritis patients, and that long-term studies should be conducted.[10] Not counting the unpublished, no control group trial by Lawrence, these two articles are the only clinical trial support for MSM for osteoarthritis.
- Seasonal Allergic Rhinitis: Barrager et al. evaluated the efficacy of MSM for hayfever.[11] Fifty-five subjects consumed 2,600 mg of MSM per day for 30 days. The study was not blinded. A significant improvement in symptoms was observed compared to those taking a placebo. No significant changes were observed in two indicators of inflammation (C-reactive protein and immunoglobulin E levels). The authors suggest that MSM is safe for short-term use and recommend that a larger, double-blind study be performed to establish its usefulness in treating symptoms of seasonal allergic rhinitis.
- Interstitial cystitis: In 1978, the FDA approved dimethyl sulfoxide (DMSO) for instillation into the bladder as a treatment for interstitial cystitis. Since DMSO is metabolized to MSM by the body, it is possible that MSM is the active ingredient in DMSO treatments.[12]
- Snoring: Blum & Blum conducted a randomized, double-blind, placebo controlled clinical trial of an MSM-containing throat spray for snoring.[13]
Pharmacology and toxicity
The LD50 (dose at which 50% of test subjects are killed) of MSM is greater than 17.5 grams per kilogram of body weight. In rats, no adverse events were observed after daily doses of 2 g MSM per kg of body weight. In a 90-day follow-up study rats received daily MSM doses of 1.5 g/kg, and no changes were observed in terms of symptoms, blood chemistry, or gross pathology.[14]
Nuclear magnetic resonance (NMR) studies have demonstrated that oral doses of MSM are absorbed into the blood and cross the blood-brain barrier.[15][16] An NMR study has also found detectable levels of MSM normally present in the blood and cerebrospinal fluid, suggesting that it derives from dietary sources, intestinal bacterial metabolism, and the body's endogenous methanethiol metabolism.[17]
The published clinical trials of MSM did not report any serious side-effects of treatment, but there are no peer-reviewed data on the effects of long-term use in humans.
See also
References
- ^ Various Names for MSM Retrieved June 8, 2009.
- ^ Georges Hareau and Philip Kocienski, "Dimethyl Sulfone" in Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley & Sons. DOI: 10.1002/047084289X.rd371
- ^ Morton JI, Siegel BV. Effects of oral dimethyl sulfoxide and dimethyl sulfone on murine autoimmune lymphoproliferative disease. Proc Soc Exp Biol Med 1986;183:227–30. PMID 3489943
- ^ Murav'ev IuV, Venikova MS, Pleskovskaia GN, et al. [Effect of dimethyl sulfoxide and dimethyl sulfone on a destructive process in the joints of mice with spontaneous arthritis]. Patol Fiziol Eksp Ter 1991;(2):37–9 [in Russian]. PMID 1881708
- ^ Williams KIH, Burstein SH, Layne DS. Dimethyl sulfone: isolation from cows’ milk. Proc Soc Exp Biol Med 1966;122:865–6. PMID 5918965
- ^ Kocsis JJ, Harkaway S, Snyder R. Biological effects of the metabolites of dimethyl sulfoxide. Ann N Y Acad Sci 1975;243:104–9. PMID 1055534
- ^ MSM the Definitive Guide: The Nutritional Breakthrough for Arthritis, Allergies and More. Freedom Press. 2003. ISBN 9781893910225.
- ^ "Pharmacists review the effectiveness, benefits and side effects of MSM".
- ^ Usha PR, Naidu MUR. Randomised, double-blind, parallel, placebo-controlled study of oral glucosamine, methylsulfonylmethane and their combination in osteoarthritis. Clin Drug Invest 2004;24(6):353–63
- ^ Kim LS, Axelrod LJ, Howard P, Buratovich N, Waters RF. Efficacy of methylsulfonylmethane (MSM) in osteoarthritis pain of the knee: a pilot clinical trial. Osteoarthritis Cartilage 2006;14(3):286–94. PMID 16309928
- ^ Barrager E, Veltmann JR, Schauss AG, Schiller RN. A multi-centered, open label trial on the safety and efficacy of methylsulfonylmethane in the treatment of seasonal allergic rhinitis. J Altern Complement Med 2002;8:167–74. PMID 12006124
- ^ Childs SJ. Dimethyl sulfone (DMSO2) in the treatment of interstitial cystitis. Urol Clin North Am 1994;21:85–8. PMID 8284850
- ^ Blum JM, Blum RI. The effect of methylsulfonylmethane (MSM) in the control of snoring. Integrative Medicine 2004;3(6)24-30
- ^ Horváth K, Noker PE, Somfai-Relle S, et al. Toxicity of methylsulfonylmethane in rats. Food Chem Toxicol 2002;40:1459–62. PMID 12387309
- ^ Rose SE, Chalk JB, Galloway GJ, Doddrell DM. Detection of dimethyl sulfone in the human brain by in vivo proton magnetic resonance spectroscopy. Magn Reson Imaging 2000;18:95–8. PMID 10642107
- ^ Lin A, Nguy CH, Shic F, Ross BD. Accumulation of methylsulfonylmethane in the human brain: identification by multinuclear magnetic resonance spectroscopy. Toxicol Lett 2001;123:169–77. PMID 11641045
- ^ Engelke UF, Tangerman A, Willemsen MA, Moskau D, Loss S, Mudd SH, Wevers RA. Dimethyl sulfone in human cerebrospinal fluid and blood plasma confirmed by one-dimensional (1)H and two-dimensional (1)H-(13)C NMR. NMR Biomed 2005 Aug;18(5):331-6. PMID 15996001
External links
- Quackwatch opinion about Methylsulfonylmethane (MSM) -- clinical dietician skeptical of MSM's marketing claims