Evoxine: Difference between revisions
Appearance
Content deleted Content added
Citation bot (talk | contribs) m Citations: [215] added: doi, last4, first4, last5, first5, last6, first6. Tweaked: last1, first1, last2, first2, last3, first3, year, title, journal, volume, issue, pages. Rjwilmsi |
m −Category:Furoquinolines; +Category:Furans; +Category:Quinolines using HotCat |
||
| Line 35: | Line 35: | ||
[[Category:Hypnotics]] |
[[Category:Hypnotics]] |
||
| ⚫ | |||
[[Category:Phenol ethers]] |
[[Category:Phenol ethers]] |
||
[[Category:Alcohols]] |
[[Category:Alcohols]] |
||
| ⚫ | |||
[[Category:Quinolines]] |
|||
Revision as of 12:58, 15 February 2011
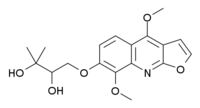 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H12O2 |
| Molar mass | 347.362 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
Evoxine (Haploperine) is an alkaloid with hypnotic and sedative effects. It is found naturally in a variety of Australian and African plants including Evodia xanthoxyloides[1] and Teclea gerrardii.[2]
References
- ^ Eastwood, FW; Hughes, GK; Ritchie, E. (1954). "Alkaloids of the Australian Rutaceae: Evodia xanthoxyloides F.Muell. IV. The structures of Evoxine and Evoxoidine". Australian Journal of Chemistry. 7 (1): 87–98. doi:10.1071/CH9540087.
- ^ Waffo, AF; Coombes, PH; Crouch, NR; Mulholland, DA; El Amin, SM; Smith, PJ (2007). "Acridone and furoquinoline alkaloids from Teclea gerrardii (Rutaceae: Toddalioideae) of southern Africa". Phytochemistry. 68 (5): 663–7. doi:10.1016/j.phytochem.2006.10.011. PMID 17174364.
