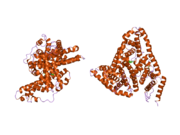Human serum albumin: Difference between revisions
logic |
→Measurement: general grammar fixes: mostly fixed/removed extraneous capitalization and punctuation |
||
| Line 41: | Line 41: | ||
==Measurement== |
==Measurement== |
||
Plasma albumin is a component of |
Plasma albumin is a component of liver function tests (LFTs), but may be ordered separately. Albumin can be measured in serum (yellow-top tube), plain tube with no additives (red-top tube), or heparin plasma (green-top tube). The reference interval is 36 - 52 g/L. (upper limit increased from 47 g/L on the 15th June 2007). One of the methods used is [[bromocresol green]] on a [[Roche Modular]] or [[Olympus AU2700]] analyser. |
||
==Reference ranges== |
==Reference ranges== |
||
Revision as of 06:24, 8 March 2011
| albumin | |||||||
|---|---|---|---|---|---|---|---|
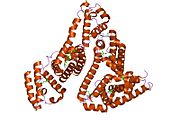
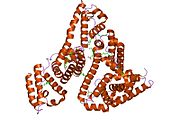
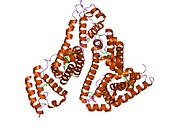
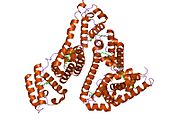
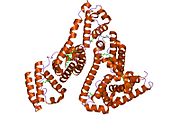
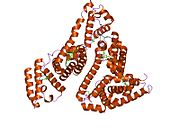
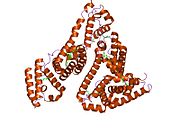
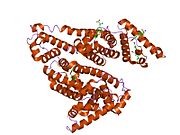
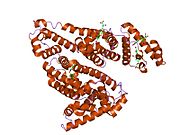
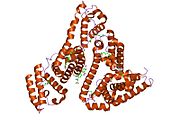
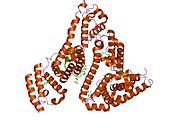
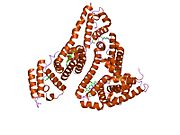
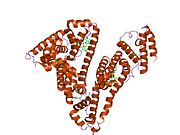
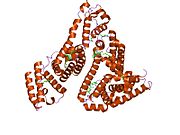
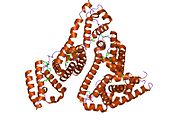
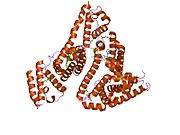
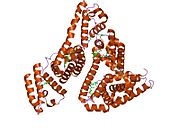
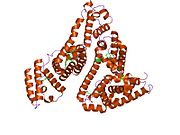
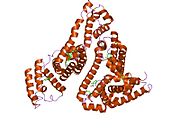
 PDB rendering based on 1e7h. | |||||||
| Identifiers | |||||||
| Symbol | ALB | ||||||
| NCBI gene | 213 | ||||||
| HGNC | 399 | ||||||
| OMIM | 103600 | ||||||
| PDB | 1E7H | ||||||
| RefSeq | NM_000477 | ||||||
| UniProt | P02768 | ||||||
| Other data | |||||||
| Locus | Chr. 4 q13.3 | ||||||
| |||||||
Human serum albumin is the most abundant protein in human blood plasma. It is produced in the liver. Albumin comprises about half of the blood serum protein. It is soluble and monomeric.
The gene for albumin is located on chromosome 4 and mutations in this gene can result in various anomalous proteins. The human albumin gene is 16,961 nucleotides long from the putative ‘cap’ site to the first poly(A) addition site. It is split into 15 exons that are symmetrically placed within the 3 domains thought to have arisen by triplication of a single primordial domain.
Albumin is synthesized in the liver as preproalbumin, which has an N-terminal peptide that is removed before the nascent protein is released from the rough endoplasmic reticulum. The product, proalbumin, is in turn cleaved in the Golgi vesicles to produce the secreted albumin.
The reference range for albumin concentrations in blood is 3.4 to 5.4 g/dL.[1] It has a serum half-life of approximately 20 days. It has a molecular mass of 67 kDa.
Function
- Maintains oncotic pressure
- Transports thyroid hormones
- Transports other hormones, in particular, ones that are fat-soluble
- Transports fatty acids ("free" fatty acids) to the liver
- Transports unconjugated bilirubin
- Transports many drugs; serum albumin levels can affect the half-life of drugs
- Competitively binds calcium ions (Ca2+)
- Buffers pH
- Serum albumin, as a negative acute-phase protein, is down-regulated in inflammatory states. As such, it is not a valid marker of nutritional status; rather, it is a marker in inflammatory states
- Prevents photodegradation of folic acid
Measurement
Plasma albumin is a component of liver function tests (LFTs), but may be ordered separately. Albumin can be measured in serum (yellow-top tube), plain tube with no additives (red-top tube), or heparin plasma (green-top tube). The reference interval is 36 - 52 g/L. (upper limit increased from 47 g/L on the 15th June 2007). One of the methods used is bromocresol green on a Roche Modular or Olympus AU2700 analyser.
Reference ranges

Pathology
Hypoalbuminemia
Low blood albumin levels (hypoalbuminemia) can be caused by:
- Liver disease; cirrhosis of the liver is most common
- Excess excretion by the kidneys (as in nephrotic syndrome)
- Excess loss in bowel (protein-losing enteropathy, e.g., Menetrier's)
- Burns (plasma loss in the absence of skin barrier)
- Redistribution (hemodilution [as in pregnancy], increased vascular permeability or decreased lymphatic clearance)
- Acute disease states (referred to as a negative acute-phase protein)
- Mutation causing analbuminemia (very rare)
Hyperalbuminemia
Typically, this condition is a sign of severe or chronic dehydration. Chronic dehydration needs to be treated with zinc as well as with water. Zinc reduces cell swelling caused by increased intake of water (hypotonicity) and also increases retention of salt. In the dehydrated state, the body has too high an osmolarity and, it appears, discards zinc to prevent this. Zinc also regulates transport of the cellular osmolyte taurine, and albumin is known to increase cellular taurine absorption. Zinc has been shown to increase retinol (vitamin A) production from beta-carotene, and in lab experiments retinol reduced human albumin production.[2] It is possible that a retinol (vitamin A) deficiency alone could cause albumin levels to become raised. Patients recovering from chronic dehydration may develop dry eyes as the body uses up its vitamin A store. It is interesting to note that retinol causes cells to swell with water (this is likely one reason that too much vitamin A is toxic).[3] Hyperalbuminemia is also associated with high protein diets.[4]
Glycosylation
It has been known for a long time that human blood proteins like hemoglobin[5] and serum albumin[6][7] may undergo a slow non-enzymatic glycation, mainly by formation of a Schiff base between ε-amino groups of lysine (and sometimes arginine) residues and glucose molecules in blood (Maillard reaction). This reaction can be inhibited in the presence of antioxidant agents.[8] Although this reaction may happen normally,[6] elevated glycoalbumin is observed in diabetes mellitus.[7]
Glycation has the potential to alter the biological structure and function of the serum albumin protein.[9][10][11][12]
Moreover, the glycation can result in the formation of Advanced Glycosylation End-Products (AGE), which result in abnormal biological effects. Accumulation of AGEs leads to tissue damage via alteration of the structures and functions of tissue proteins, stimulation of cellular responses, through receptors specific for AGE-proteins, and generation of reactive oxygen intermediates. AGEs also react with DNA, thus causing mutations and DNA transposition. Thermal processing of proteins and carbohydrates brings major changes in allergenicity. AGEs are antigenic and represent many of the important neoantigens found in cooked or stored foods.[13] They also interfere with the normal product of nitric oxide in cells.[14]
Although there are several lysine and arginine residues in the serum albumin structure, very few of them can take part in the glycation reaction.[7][15] It is not clear exactly why only these residues are glycated in serum albumin, but it is suggested that non-covalent binding of glucose to serum albumin prior to the covalent bond formation might be the reason.[16]
Loss via kidneys
In the healthy kidney, albumin’s size and negative electric charge exclude it from excretion in the glomerulus. This is not always the case, as in some diseases including diabetic nephropathy, a major complication of uncontrolled diabetes in which proteins can cross the glomerulus. The lost albumin can be detected by a simple urine test.[17] Depending on the amount of albumin lost, a patient may have normal renal function, microalbuminuria, or albuminuria.
Amino acid sequence
The approximate sequence of human serum albumin is:
MKWVTFISLL FLFSSAYSRG VFRRDAHKSE VAHRFKDLGE ENFKALVLIA FAQYLQQCPF EDHVKLVNEV TEFAKTCVAD ESAENCDKSL HTLFGDKLCT VATLRETYGE MADCCAKQEP ERNECFLQHK DDNPNLPRLV RPEVDVMCTA FHDNEETFLK KYLYEIARRH PYFYAPELLF FAKRYKAAFT ECCQAADKAA CLLPKLDELR DEGKASSAKQ RLKCASLQKF GERAFKAWAV ARLSQRFPKA EFAEVSKLVT DLTKVHTECC HGDLLECADD RADLAKYICE NQDSISSKLK ECCEKPLLEK SHCIAEVEND EMPADLPSLA ADFVESKDVC KNYAEAKDVF LGMFLYEYAR RHPDYSVVLL LRLAKTYETT LEKCCAAADP HECYAKVFDE FKPLVEEPQN LIKQNCELFE QLGEYKFQNA LLVRYTKKVP QVSTPTLVEV SRNLGKVGSK CCKHPEAKRM PCAEDYLSVV LNQLCVLHEK TPVSDRVTKC CTESLVNRRP CFSALEVDET YVPKEFNAET FTFHADICTL SEKERQIKKQ TALVELVKHK PKATKEQLKA VMDDFAAFVE KCCKADDKET CFAEEGKKLV AASQAALGL
The italicized first 24 amino acids are signal and propeptide portions not observed in the transcribed, translated, and transported protein but present in the gene. There are 609 amino acids in this sequence with only 585 amino acids in the final product observed in the blood.
Interactions
Human serum albumin has been shown to interact with FCGRT.[18]
See also
References
- ^ "Albumin - serum: MedlinePlus Medical Encyclopedia". Nlm.nih.gov. Retrieved 2010-05-12.
- ^ Masaki T, Matsuura T, Ohkawa K, Miyamura T, Okazaki I, Watanabe T, Suzuki T (2006). "All-trans retinoic acid down-regulates human albumin gene expression through the induction of C/EBPbeta-LIP". Biochem. J. 397 (2): 345–53. doi:10.1042/BJ20051863. PMC 1513275. PMID 16608438.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Pasantes-Morales H, Wright CE, Gaull GE (1984). "Protective effect of taurine, zinc and tocopherol on retinol-induced damage in human lymphoblastoid cells". J. Nutr. 114 (12): 2256–61. PMID 6502269.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mutlu EA, Keshavarzian A, Mutlu GM (2006). "Hyperalbuminemia and elevated transaminases associated with high-protein diet". Scand. J. Gastroenterol. 41 (6): 759–60. doi:10.1080/00365520500442625. PMID 16716979.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Rajbar S (1968). "An abnormal hemoglobin in red cells of diabetics". Clin. Chim. Acta. 22 (2): 296–8. doi:10.1016/0009-8981(68)90372-0. PMID 5687098.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Day JF, Thorpe SR, Baynes JW (1979). "Nonenzymatically glucosylated albumin. In vitro preparation and isolation from normal human serum". J. Biol. Chem. 254 (3): 595–7. PMID 762083.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Iberg N, Flückiger R (1986). "Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites". J. Biol. Chem. 261 (29): 13542–5. PMID 3759977.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Jakus V, Hrnciarová M, Cársky J, Krahulec B, Rietbrock N (1999). "Inhibition of nonenzymatic protein glycation and lipid peroxidation by drugs with antioxidant activity". Life Sci. 65 (18–19): 1991–3. doi:10.1016/S0024-3205(99)00462-2. PMID 10576452.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mohamadi-Nejad A, Moosavi-Movahedi AA, Hakimelahi GH, Sheibani N (2002). "Thermodynamic analysis of human serum albumin interactions with glucose: insights into the diabetic range of glucose concentration". Int. J. Biochem. Cell Biol. 34 (9): 1115–24. doi:10.1016/S1357-2725(02)00031-6. PMID 12009306.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Shaklai N, Garlick RL, Bunn HF (1984). "Nonenzymatic glycosylation of human serum albumin alters its conformation and function". J. Biol. Chem. 259 (6): 3812–7. PMID 6706980.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mendez DL, Jensen RA, McElroy LA, Pena JM, Esquerra RM (2005). "The effect of non-enzymatic glycation on the unfolding of human serum albumin". Arch. Biochem. Biophys. 444 (2): 92–9. doi:10.1016/j.abb.2005.10.019. PMID 16309624.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mohamadi-Nejada A, Moosavi-Movahedi AA,Safariana S, Naderi-Maneshc MH, Ranjbarc B, Farzamid B, Mostafavie H, Larijanif MB, Hakimelahi GH, A (2002). "The thermal analysis of nonezymatic glycosylation of human serum albumin: differential scanning calorimetry and circular dichroism studies". Thermochimica Acta. 389 (1–2): 141–151. doi:10.1016/S0040-6031(02)00006-0.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kańska U, Boratyński J (2002). "Thermal glycation of proteins by D-glucose and D-fructose". Arch. Immunol. Ther. Exp. (Warsz.). 50 (1): 61–6. PMID 11916310.
- ^ Rojas A, Romay S, González D, Herrera B, Delgado R, Otero K (2000). "Regulation of endothelial nitric oxide synthase expression by albumin-derived advanced glycosylation end products". Circ. Res. 86 (3): E50–4. PMID 10679490.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Garlick RL, Mazer JS (1983). "The principal site of nonenzymatic glycosylation of human serum albumin in vivo". J. Biol. Chem. 258 (10): 6142–6. PMID 6853480.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Marashi SA, Safarian S, Moosavi-Movahedi AA (2005). "Why major nonenzymatic glycation sites of human serum albumin are preferred to other residues?". Med. Hypotheses. 64 (4): 881. doi:10.1016/j.mehy.2004.11.007. PMID 15694713.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Microalbumin Urine Test
- ^ Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL (2003). "The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan". J. Exp. Med. 197 (3): 315–22. doi:10.1084/jem.20021829. PMC 2193842. PMID 12566415.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
Further reading
- Komatsu T, Nakagawa A, Curry S; et al. (2009). "The role of an amino acid triad at the entrance of the heme pocket in human serum albumin for O(2) and CO binding to iron protoporphyrin IX". Org. Biomol. Chem. 7 (18): 3836–41. doi:10.1039/b909794e. PMID 19707690.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Milojevic J, Raditsis A, Melacini G (2009). "Human serum albumin inhibits Abeta fibrillization through a "monomer-competitor" mechanism". Biophys. J. 97 (9): 2585–94. doi:10.1016/j.bpj.2009.08.028. PMC 2770600. PMID 19883602.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Silva AM, Hider RC (2009). "Influence of non-enzymatic post-translation modifications on the ability of human serum albumin to bind iron. Implications for non-transferrin-bound iron speciation". Biochim. Biophys. Acta. 1794 (10): 1449–58. doi:10.1016/j.bbapap.2009.06.003. PMID 19505594.
- Otosu T, Nishimoto E, Yamashita S (2010). "Multiple conformational state of human serum albumin around single tryptophan residue at various pH revealed by time-resolved fluorescence spectroscopy". J. Biochem. 147 (2): 191–200. doi:10.1093/jb/mvp175. PMID 19884191.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Blindauer CA, Harvey I, Bunyan KE; et al. (2009). "Structure, properties, and engineering of the major zinc binding site on human albumin". J. Biol. Chem. 284 (34): 23116–24. doi:10.1074/jbc.M109.003459. PMC 2755717. PMID 19520864.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - Juárez J, Lápez SG, Cambán A; et al. (2009). "Influence of electrostatic interactions on the fibrillation process of human serum albumin". J Phys Chem B. 113 (30): 10521–9. doi:10.1021/jp902224d. PMID 19572666.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Fu BL, Guo ZJ, Tian JW; et al. (2009). "[Advanced glycation end products induce expression of PAI-1 in cultured human proximal tubular epithelial cells through NADPH oxidase dependent pathway]". Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 25 (8): 674–7. PMID 19664386.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Ascenzi P, di Masi A, Coletta M; et al. (2009). "Ibuprofen impairs allosterically peroxynitrite isomerization by ferric human serum heme-albumin". J. Biol. Chem. 284 (45): 31006–17. doi:10.1074/jbc.M109.010736. PMC 2781501. PMID 19734142.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - Sowa ME, Bennett EJ, Gygi SP, Harper JW (2009). "Defining the human deubiquitinating enzyme interaction landscape". Cell. 138 (2): 389–403. doi:10.1016/j.cell.2009.04.042. PMC 2716422. PMID 19615732.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Curry S (2002). "Beyond expansion: structural studies on the transport roles of human serum albumin". Vox Sang. 83 Suppl 1: 315–9. PMID 12617161.
- Guo S, Shi X, Yang F; et al. (2009). "Structural basis of transport of lysophospholipids by human serum albumin". Biochem. J. 423 (1): 23–30. doi:10.1042/BJ20090913. PMID 19601929.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - de Jong PE, Gansevoort RT (2009). "Focus on microalbuminuria to improve cardiac and renal protection". Nephron Clin Pract. 111 (3): c204–10, discussion c211. doi:10.1159/000201568. PMID 19212124.
- Page TA, Kraut ND, Page PM; et al. (2009). "Dynamics of loop 1 of domain I in human serum albumin when dissolved in ionic liquids". J Phys Chem B. 113 (38): 12825–30. doi:10.1021/jp904475v. PMID 19711930.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Roche M, Rondeau P, Singh NR; et al. (2008). "The antioxidant properties of serum albumin". FEBS Lett. 582 (13): 1783–7. doi:10.1016/j.febslet.2008.04.057. PMID 18474236.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Wyatt AR, Wilson MR (2010). "Identification of human plasma proteins as major clients for the extracellular chaperone clusterin". J. Biol. Chem. 285 (6): 3532–9. doi:10.1074/jbc.M109.079566. PMC 2823492. PMID 19996109.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Cui FL, Yan YH, Zhang QZ; et al. (2010). "A study on the interaction between 5-Methyluridine and human serum albumin using fluorescence quenching method and molecular modeling". J Mol Model. 16 (2): 255–62. doi:10.1007/s00894-009-0548-4. PMID 19588173.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Caridi G, Dagnino M, Simundic AM; et al. (2010). "Albumin Benkovac (c.1175 A > G; p.Glu392Gly): a novel genetic variant of human serum albumin". Transl Res. 155 (3): 118–9. doi:10.1016/j.trsl.2009.10.001. PMID 20171595.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Deeb O, Rosales-Hernández MC, Gámez-Castro C; et al. (2010). "Exploration of human serum albumin binding sites by docking and molecular dynamics flexible ligand-protein interactions". Biopolymers. 93 (2): 161–70. doi:10.1002/bip.21314. PMID 19785033.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Karahan SC, Koramaz I, Altun G; et al. (2010). "Ischemia-modified albumin reduction after coronary bypass surgery is associated with the cardioprotective efficacy of cold-blood cardioplegia enriched with N-acetylcysteine: a preliminary study". Eur Surg Res. 44 (1): 30–6. doi:10.1159/000262324. PMID 19955769.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Jin C, Lu L, Zhang RY; et al. (2009). "Association of serum glycated albumin, C-reactive protein and ICAM-1 levels with diffuse coronary artery disease in patients with type 2 diabetes mellitus". Clin. Chim. Acta. 408 (1–2): 45–9. doi:10.1016/j.cca.2009.07.003. PMID 19615354.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link)