Sodium hypochlorite: Difference between revisions
logic, grammar, syntax |
|||
| Line 68: | Line 68: | ||
Hypochlorite was first produced in 1789 by [[Claude Louis Berthollet]] in his laboratory on the quay [[Javel - André Citroën (Paris Métro)|Javel]] in [[Paris]], France, by passing [[chlorine]] gas through a solution of sodium carbonate. The resulting liquid, known as "''Eau de Javel''" ("Javel water"), was a weak solution of sodium hypochlorite. However, this process was not very efficient, and alternate production methods were sought. One such method involved the extraction of chlorinated lime (known as bleaching powder) with sodium carbonate to yield low levels of available chlorine. This method was commonly used to produce hypochlorite solutions for use as a hospital antiseptic that was sold under the trade names "Eusol" and "Dakin's solution". |
Hypochlorite was first produced in 1789 by [[Claude Louis Berthollet]] in his laboratory on the quay [[Javel - André Citroën (Paris Métro)|Javel]] in [[Paris]], France, by passing [[chlorine]] gas through a solution of sodium carbonate. The resulting liquid, known as "''Eau de Javel''" ("Javel water"), was a weak solution of sodium hypochlorite. However, this process was not very efficient, and alternate production methods were sought. One such method involved the extraction of chlorinated lime (known as bleaching powder) with sodium carbonate to yield low levels of available chlorine. This method was commonly used to produce hypochlorite solutions for use as a hospital antiseptic that was sold under the trade names "Eusol" and "Dakin's solution". |
||
Near the end of the nineteenth century, E. S. Smith patented a method of hypochlorite production involving hydrolysis of [[brine]] to produce [[caustic soda]] and chlorine gas, which then mixed to form hypochlorite.{{Citation needed|date=March 2009}} Both electric power and brine solution were in cheap supply at this time, and various enterprising marketers took advantage of this situation to satisfy the market's demand for hypochlorite. Bottled solutions of hypochlorite were sold under numerous trade names. |
Near the end of the nineteenth century, E. S. Smith patented a method of hypochlorite production involving electrolysis<!--hydrolysis--> of [[brine]] to produce [[caustic soda]] and chlorine gas, which then mixed to form hypochlorite.{{Citation needed|date=March 2009}} Both electric power and brine solution were in cheap supply at this time, and various enterprising marketers took advantage of this situation to satisfy the market's demand for hypochlorite. Bottled solutions of hypochlorite were sold under numerous trade names. |
||
Today, an improved version of this method, known as the Hooker process, is the only large scale industrial method of sodium hypochlorite production. In this process sodium hypochlorite (NaClO) and [[sodium chloride]] (NaCl) are formed when chlorine is passed into cold and dilute [[sodium hydroxide]] solution. It is prepared industrially by [[electrolysis]] with minimal separation between the [[anode]] and the [[cathode]]. The solution must be kept below 40 °C (by cooling coils) to prevent the undesired formation of [[sodium chlorate]]. |
Today, an improved version of this method, known as the Hooker process, is the only large scale industrial method of sodium hypochlorite production. In this process sodium hypochlorite (NaClO) and [[sodium chloride]] (NaCl) are formed when chlorine is passed into cold and dilute [[sodium hydroxide]] solution. It is prepared industrially by [[electrolysis]] with minimal separation between the [[anode]] and the [[cathode]]. The solution must be kept below 40 °C (by cooling coils) to prevent the undesired formation of [[sodium chlorate]]. |
||
Revision as of 07:27, 8 March 2011
| |||
| |||
| Names | |||
|---|---|---|---|
| Other names
Sodium chlorate(I)
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.028.790 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1791 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| NaClO | |||
| Molar mass | 74.44 g/mol | ||
| Appearance | white solid | ||
| Density | 1.11 g/cm3 | ||
| Melting point | 18 °C (pentahydrate) | ||
| Boiling point | 101 °C (decomp.) | ||
| 29.3 g/100ml (0 °C) | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Other anions
|
Sodium chloride Sodium chlorite Sodium chlorate Sodium perchlorate | ||
Other cations
|
Lithium hypochlorite Calcium hypochlorite | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
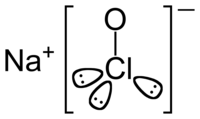
Sodium hypochlorite is a chemical compound with the formula NaClO. Sodium hypochlorite solution, commonly known as bleach, is frequently used as a disinfectant or a bleaching agent.
Production
Hypochlorite was first produced in 1789 by Claude Louis Berthollet in his laboratory on the quay Javel in Paris, France, by passing chlorine gas through a solution of sodium carbonate. The resulting liquid, known as "Eau de Javel" ("Javel water"), was a weak solution of sodium hypochlorite. However, this process was not very efficient, and alternate production methods were sought. One such method involved the extraction of chlorinated lime (known as bleaching powder) with sodium carbonate to yield low levels of available chlorine. This method was commonly used to produce hypochlorite solutions for use as a hospital antiseptic that was sold under the trade names "Eusol" and "Dakin's solution".
Near the end of the nineteenth century, E. S. Smith patented a method of hypochlorite production involving electrolysis of brine to produce caustic soda and chlorine gas, which then mixed to form hypochlorite.[citation needed] Both electric power and brine solution were in cheap supply at this time, and various enterprising marketers took advantage of this situation to satisfy the market's demand for hypochlorite. Bottled solutions of hypochlorite were sold under numerous trade names.
Today, an improved version of this method, known as the Hooker process, is the only large scale industrial method of sodium hypochlorite production. In this process sodium hypochlorite (NaClO) and sodium chloride (NaCl) are formed when chlorine is passed into cold and dilute sodium hydroxide solution. It is prepared industrially by electrolysis with minimal separation between the anode and the cathode. The solution must be kept below 40 °C (by cooling coils) to prevent the undesired formation of sodium chlorate.
- Cl2 + 2 NaOH → NaCl + NaClO + H2O
Sodium hydroxide and chlorine are commercially produced by the chloralkali process, and there is no need to isolate them to prepare sodium hypochlorite.
Hence, chlorine is simultaneously reduced and oxidized; this process is known as disproportionation.
The commercial solutions always contain significant amounts of sodium chloride (common salt) as the main by-product, as seen in the equation above.
Sodium hypochlorite can be also made by electrolyzing saturated sodium chloride solution, and the product can be tested by dropping hydrochloric acid to determine whether it is successfully synthesized.
Packaging and sale
Household bleach sold for use in laundering clothes is a 3-6% solution of sodium hypochlorite at the time of manufacture. Strength varies from one formulation to another and gradually decreases with long storage.
A 12% solution is widely [1] used in waterworks for the chlorination of water, and a 15% solution is more commonly[1] used for disinfection of waste water in treatment plants. High-test hypochlorite (HTH) is sold for chlorination of swimming pools and contains approximately 30% calcium hypochlorite. The crystalline salt is also sold for the same use; this salt usually contains less than 50% of calcium hypochlorite. However, the level of active chlorine may be much higher.
It can also be found on store shelves in Daily Sanitizing Sprays, as the sole active ingredient at 0.0095%.[2]
Reactions
Sodium hypochlorite reacts with metals gradually, such as zinc, to produce the metal oxide or hydroxide:
- NaClO + Zn → ZnO + NaCl
It reacts with hydrochloric acid to release chlorine gas:
- NaClO + 2 HCl → Cl2 + H2O + NaCl
It reacts with other acids, such as acetic acid, to release hypochlorous acid:
- NaClO + CH3COOH → HClO + CH3COONa
It decomposes when heated or evaporated to form sodium chlorate and sodium chloride:
- 3 NaClO → NaClO3 + 2 NaCl
In reaction with hydrogen peroxide it gives off molecular oxygen:
- NaClO + H2O2 → H2O + NaCl + O2↑
Uses
Bleaching
Household bleach is, in general, a solution containing 4-6% sodium hypochlorite and 0.01-0.05% sodium hydroxide; the sodium hydroxide is used to delay the breakdown of sodium hypochlorite into sodium chloride and sodium chlorate.[3]
In household form, sodium hypochlorite is used for removal of stains from laundry. It is particularly effective on cotton fiber, which stains easily but bleaches well. Usually 50 to 250 mL of bleach per load is recommended for a standard-size washer. The properties of household bleach that make it effective for removing stains also result in cumulative damage to organic fibers such as cotton, and the useful lifespan of these materials will be shortened with regular bleaching. The sodium hydroxide (NaOH) that is also found in household bleach (as noted later) causes fiber degradation as well. It is not volatile, and residual amounts of NaOH not rinsed out will continue slowly degrading organic fibers in the presence of humidity. For these reasons, if stains are localized, spot treatments should be considered whenever possible. With safety precautions, post-treatment with vinegar (or another weak acid) will neutralize the NaOH, and volatilize the chlorine from residual hypochlorite. Old t-shirts and cotton sheets that rip easily demonstrate the costs of laundering with household bleach. Hot water increases the activity of the bleach, owing to the increased kinetic energy of the molecules.
Disinfection
A weak solution of 2% household bleach in warm water is used to sanitize smooth surfaces prior to brewing of beer or wine. Surfaces must be rinsed to avoid imparting flavors to the brew; these chlorinated byproducts of sanitizing surfaces are also harmful.
US Government regulations (21 CFR Part 178) allow food processing equipment and food contact surfaces to be sanitized with solutions containing bleach, provided that the solution is allowed to drain adequately before contact with food, and that the solutions do not exceed 200 parts per million (ppm) available chlorine (for example, one tablespoon of typical household bleach containing 5.25% sodium hypochlorite, per gallon of water). If higher concentrations are used, the surface must be rinsed with potable water after sanitizing.
A 1-in-5 dilution of household bleach with water (1 part bleach to 4 parts water) is effective against many bacteria and some viruses, and is often the disinfectant of choice in cleaning surfaces in hospitals (Primarily in the United States). The solution is corrosive, and needs to be thoroughly removed afterwards, so the bleach disinfection is sometimes followed by an ethanol disinfection. Even "scientific-grade", commercially produced disinfection solutions such as Virocidin-X usually have sodium hypochlorite as their sole active ingredient, though they also contain surfactants (to prevent beading) and fragrances (to conceal the bleach smell) [4].
Water treatment
For shock chlorination of wells or water systems, a 3% solution of household bleach is used. For larger systems, sodium hypochlorite is more practical because lower rates can be used. The alkalinity of the sodium hypochlorite solution also causes the precipitation of minerals such as calcium carbonate, so that the shock chlorination is often accompanied by a clogging effect. The precipitate also preserves bacteria, making this practice somewhat less effective.
Sodium hypochlorite has been used for the disinfection of drinking water. A concentration equivalent to about 1 liter of household bleach per 4000 liters of water is used. The exact amount required depends on the water chemistry, temperature, contact time, and presence or absence of sediment. In large-scale applications, residual chlorine is measured to titrate the proper dosing rate. For emergency disinfection, the United States Environmental Protection Agency recommends the use of 2 drops of 5%ac household bleach per litre of water. If the treated water does not smell of bleach, 2 more drops are to be added.
The use of chlorine-based disinfectants in domestic water, although widespread, has led to some controversy due to the formation of small quantities of harmful byproducts such as chloroform.
An alkaline solution (pH 11.0) of sodium hypochlorite is used to treat dilute (< 1 g/L) cyanide wastewater, e.g., rinsewater from an electroplating shop. In batch treatment operations, sodium hypochlorite has been used to treat more concentrated cyanide wastes, such as silver cyanide plating solutions. A well-mixed solution is fully treated when an excess of chlorine is detected.
Endodontics
Sodium hypochlorite is now used in endodontics during root canal treatments. It is the medicament of choice due to its efficacy against pathogenic organisms and pulp digestion. In previous times, Henry Drysdale Dakin's solution (0.5%) had been used. Its concentration for use in endodontics today varies from 0.5% to 5.25%. At low concentrations it will dissolve mainly necrotic tissue; whereas at higher concentrations tissue dissolution is better but it also dissolves vital tissue, a generally undesirable effect. It has been shown that clinical effectiveness does not increase conclusively for concentrations higher than 1% [5].
Oxidation
Household bleach, with a phase-transfer catalyst, has been reported to oxidize alcohols to the corresponding carbonyl compound.[6]
Mechanism of action
See Hypochlorous acid.
Safety
Sodium hypochlorite is a strong oxidizer. Oxidation reactions are corrosive, solutions burn skin and cause eye damage, in particular, when used in concentrated forms. However, as recognized by the NFPA, only solutions containing more than 40% sodium hypochlorite by weight are considered hazardous oxidizers. Solutions less than 40% are classified as a moderate oxidizing hazard (NFPA 430, 2000).
Chlorination of drinking water can oxidize organic contaminants, producing trihalomethanes (also called haloforms), which are carcinogenic.
Household bleach and pool chlorinator solutions are typically stabilized by a significant concentration of lye (caustic soda, NaOH) as part of the manufacturing reaction. Skin contact will produce caustic irritation or burns due to defatting and saponification of skin oils and destruction of tissue. The slippery feel of bleach on skin is due to this process.
Sodium thiosulfate (thio) is an effective chlorine neutralizer. Rinsing with a 5 mg/L solution, followed by washing with soap and water, quickly removes chlorine odor from the hands.
Mixing bleach with some household cleaners can be hazardous. For example, mixing an acid cleaner with sodium hypochlorite bleach generates chlorine gas. Mixing with ammonia solutions (including urine) produces chloramines.
- NH4OH + NaClO → NaOH + NH2Cl + H2O
Both chlorine gas and chloramine gas are toxic. Bleach can react violently with hydrogen peroxide and produce oxygen gas:[7]
- H2O2(aq) + NaClO(aq) → NaCl(aq) + H2O(l) + O2(g)
It is estimated that there are about 3300 accidents needing hospital treatment caused by sodium hypochlorite solutions each year in British homes (RoSPA, 2002).
One major concern arising from sodium hypochlorite use is that it tends to form chlorinated organic compounds; this can occur during household storage and use as well during industrial use.[3] For example, when household bleach and wastewater were mixed, 1-2% of the available chlorine was observed to form organic compounds.[3] As of 1994, not all the byproducts had been identified, but identified compounds include chloroform and carbon tetrachloride.[3] The estimated exposure to these chemicals from use is estimated to be within occupational exposure limits.[3]
A recent European study indicated that sodium hypochlorite and organic chemicals (e.g., surfactants, fragrances) contained in several household cleaning products can react to generate chlorinated volatile organic compounds (VOCs).[8] These chlorinated compounds are emitted during cleaning applications, some of which are toxic and probable human carcinogens. The study showed that indoor air concentrations significantly increase (8-52 times for chloroform and 1-1170 times for carbon tetrachloride, respectively, above baseline quantities in the household) during the use of bleach containing products. The increase in chlorinated volatile organic compound concentrations was the lowest for plain bleach and the highest for the products in the form of “thick liquid and gel”. The significant increases observed in indoor air concentrations of several chlorinated VOCs (especially carbon tetrachloride and chloroform) indicate that the bleach use may be a source that could be important in terms of inhalation exposure to these compounds. While the authors suggested that using these cleaning products may significantly increase the cancer risk [9], this conclusion appears to be hypothetical:
- The highest level cited for concentration of carbon tetrachloride (seemingly of highest concern) is 459 micrograms per cubic meter, translating to 0.073 ppm (part per million), or 73 ppb (part per billion). The OSHA-allowable time-weighted average concentration over an eight-hour period is 10 ppm [10], almost 140 times higher;
- The OSHA highest allowable peak concentration (5-minute exposure for five minutes in a 4-hour period) is 200 ppm[10], twice as high as the reported highest peak level (from the headspace of a bottle of a sample of bleach plus detergent).
Further studies of the use of these products and other possible exposure routes (i.e., dermal) may reveal other risks. Though the author further cited ozone depletion greenhouse effects for these gases, the very low amount of such gases, generated as prescribed, should minimize their contribution relative to other sources.
Environmental impact
Most of the sodium hypochlorite will break down into sodium chloride and sodium chlorate, but a small percentage breaks down into chlorocarbons, with chloroform and carbon tetrachloride being identified.[3] It has been estimated with market data that storage of household products in 1992 would have contributed to 12 tons of chloroform and 28 tons of carbon tetrachloride. Chloroform breaks down in the troposphere and it was estimated that about 96,000 tons of carbon tetrachloride are released annually.[3]
References
- ^ Metcalf & Eddy, Inc (1991). Wastewater Engineering: Treatment, Disposal, & Reuse 3rd Edition; pg 497
- ^ "What is in Daily Sanitizing Spray?". March 2010.
- ^ a b c d e f g Smith WT. (1994). Human and Environmental Safety of Hypochlorite. In: Proceedings of the 3rd World Conference on Detergents: Global Perspectives, pp. 183-5. Note: The author,Smith, works for Clorox.
- ^ http://www.kamscientific.com/
- ^ Zehnder M; et al. (2002). "Tissue dissolving capacity and antibacterial effect of buffered and unbuffered hypochlorite solutions". Oral Surg Oral Med Oral Pathol Oral Radio Endodon. 94 (6): 756. doi:10.1067/moe.2002.128961. PMID 12464903.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ G. A. Mirafzal and A. M. Lozeva (1998). "Phase transfer catalyzed oxidation of alcohols with sodium hypochlorite". Tetrahedron Letters. 39 (40): 7263–7266. doi:10.1016/S0040-4039(98)01584-6.
- ^ "Hydrogen Peroxide + Bleach Explanation". Retrieved 13 December 2008.
- ^ Odabasi, M., “Halogenated Volatile Organic Compounds from the Use of Chlorine-Bleach- Containing Household Products”, Environmental Science & Technology 42, 1445-1451, (2008). Available at: http://pubs.acs.org/journals/esthag/
- ^ Odabasi, M., “Halogenated Volatile Organic Compounds from the Use of Chlorine-Bleach- Containing Household Products, Slide presentation (2008). Available at: http://www.slideworld.org/ViewSlides.aspx?URL=5092
- ^ a b http://www.osha.gov/dts/chemicalsampling/data/CH_225800.html
Bibliography
- Jones, F.-L. (1972). "Chlorine poisoning from mixing household cleaners". J. Am. Med. Assoc. 222: 1312. doi:10.1001/jama.222.10.1312.
- Institut National de Recherche et de Sécurité. (2004). "Eaux et extraits de Javel. Hypochlorite de sodium en solution". Fiche toxicologique n° 157, Paris.
External links
- International Chemical Safety Card 0482 (solutions<10% active Cl)
- International Chemical Safety Card 1119 (solutions >10% active Cl)
- European Chemicals Bureau
- Institut national de recherche et de sécurité (in French)
- Home and Leisure Accident Statistics 2002 (UK RoSPA)
- Emergency Disinfection of Drinking Water (United States Environmental Protection Agency)
- Chlorinated Drinking Water (IARC Monograph)
- NTP Study Report TR-392: Chlorinated & Chloraminated Water (US NIH)
- Guidelines for the Use of Chlorine Bleach as a Sanitizer in Food Processing Operations (Oklahoma State University)



