Methylene blue: Difference between revisions
PA MD0351XXE (talk | contribs) →Medicine: Referenced the use of Methylene Blue in a urinary analgesic combination medication |
PA MD0351XXE (talk | contribs) |
||
| Line 102: | Line 102: | ||
|isbn = 0674006194}}</ref> |
|isbn = 0674006194}}</ref> |
||
Methylene blue is a component of a frequently prescribed urinary analgesic/anti-infective/anti-spasmodic known as Prosed, which also contains [[Methanamine]], [[Hyoscyamine Sulfate]], and [[Salicylate]]. A similar, more popular combination medication known as Urised (and its generic counterparts) |
Methylene blue is a component of a frequently prescribed urinary analgesic/anti-infective/anti-spasmodic known as Prosed, which also contains [[Methanamine]], [[Hyoscyamine Sulfate]], and [[Salicylate]]. A similar, more popular combination medication known as Urised (and its generic counterparts) was discontinued in 2007, quite possibly because of the way in which Methanamine was included into the formulation (and its ease of separation from the other components of the medication), and the fact that Methanamine is a precursor to [[Methamphetamine]], making it a target for clandestine use. The new formulation (Prosed) sequesters the Methanamine using a method which makes its separation nearly impossible. |
||
====Malaria==== |
====Malaria==== |
||
Revision as of 01:08, 24 April 2011
| |
| |
| Names | |
|---|---|
| IUPAC names
3,7-bis(Dimethylamino)-
phenothiazin-5-ium chloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.000.469 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H18N3SCl | |
| Molar mass | 319.85 g/mol |
| Melting point | 100-110 °C (with decomposition) |
| Boiling point | Decomposes |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
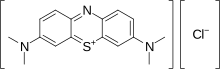
Methylene blue is a heterocyclic aromatic chemical compound with the molecular formula C16H18N3SCl. It has many uses in a range of different fields, such as biology and chemistry. At room temperature it appears as a solid, odorless, dark green powder, that yields a blue solution when dissolved in water. The hydrated form has 3 molecules of water per molecule of methylene blue.[1] Methylene blue should not be confused with methyl blue, another histology stain, new methylene blue, nor with the methyl violets often used as pH indicators.
The International Nonproprietary Name (INN) of methylene blue is methylthioninium chloride.[2][3]
Uses
Chemistry

Redox indicator
Methylene blue is widely used as a redox indicator in analytical chemistry. Solutions of this substance are blue when in an oxidizing environment, but will turn colorless if exposed to a reducing agent. The redox properties can be seen in a classical demonstration of chemical kinetics in general chemistry, the "blue bottle" experiment. Typically, a solution is made of dextrose, methylene blue, and sodium hydroxide. Upon shaking the bottle, oxygen oxidizes methylene blue, and the solution turns blue. The dextrose will gradually reduce the methylene blue to its colorless, reduced form. Hence, when the dissolved oxygen is entirely consumed, the solution will turn green.
Peroxide generator
Methylene blue is also a photosensitizer used to create singlet oxygen when exposed to both oxygen and light. It is used in this regard to make organic peroxides by a Diels-Alder reaction which is spin forbidden with normal atmospheric triplet oxygen.
Sulfide analysis
The formation of methylene blue after the reaction of hydrogen sulfide with dimethyl-p-phenylenediamine and iron(III) at pH 0.4 – 0.7 is used to determine by photometric measurements sulfide concentration in the range 0.020 to 1.50 mg/L (20 ppb to 1.5 ppm). The test is very sensitive and the blue coloration developing upon contact of the reagents with dissolved H2S is stable for 60 min. Ready-to-use kits such as the Spectroquant sulfide test[4] facilitate routine analyses. The methylene blue sulfide test is a convenient method often used in soil microbiology to quickly detect in water the metabolic activity of sulfate reducing bacteria (SRB). It must be[citation needed] well noted that in this test, methylene blue is a product of reaction and not a reagent.
The addition of a strong reducing agent, such as ascorbic acid, to a sulfide-containing solution is sometimes used to prevent sulfide oxidation from atmospheric oxygen. Although it is certainly a sound precaution for the determination of sulfide with an ion selective electrode, it might however hamper the development of the blue color if the freshly formed methylene blue is also reduced, as described here above in the paragraph on redox indicator.
Biology
In biology methylene blue is used as a dye for a number of different staining procedures, such as Wright's stain and Jenner's stain. Since it is a temporary staining technique, methylene blue can also be used to examine RNA or DNA under the microscope or in a gel: as an example, a solution of methylene blue can be used to stain RNA on hybridization membranes in northern blotting to verify the amount of nucleic acid present. While methylene blue is not as sensitive as ethidium bromide, it is less toxic and it does not intercalate in nucleic acid chains, thus avoiding interference with nucleic acid retention on hybridization membranes or with the hybridization process itself.
It can also be used as an indicator to determine if a cell such as yeast is alive or not. The blue indicator turns colorless in the presence of active enzymes, thus indicating living cells. However, if it stays blue it doesn't mean that the cell is dead - the enzymes could be inactive/denatured. Methylene blue can inhibit the respiration of the yeast as it picks up hydrogen ions made during the process and the yeast cell cannot then use those ions to release energy.
In neuroscience, methylene blue can also serve as a non-selective inhibitor of NO synthase.
Medicine
Methylene blue is a monoamine oxidase inhibitor (MAOI),[5] and if infused intravenously at doses exceeding 5 mg/kg, may precipitate serious serotonin toxicity, serotonin syndrome, if combined with any selective serotonin reuptake inhibitors (SSRIs) or other serotonin reuptake inhibitor (e.g., duloxetine, sibutramine, venlafaxine, clomipramine, imipramine).[6]
Methylene blue is also structurally similar to the chlorpromazine and the typical antipsychotics. It is the basic compound from which chlorpromazine and many other antipsychotics are made.[7]
Methylene blue is a component of a frequently prescribed urinary analgesic/anti-infective/anti-spasmodic known as Prosed, which also contains Methanamine, Hyoscyamine Sulfate, and Salicylate. A similar, more popular combination medication known as Urised (and its generic counterparts) was discontinued in 2007, quite possibly because of the way in which Methanamine was included into the formulation (and its ease of separation from the other components of the medication), and the fact that Methanamine is a precursor to Methamphetamine, making it a target for clandestine use. The new formulation (Prosed) sequesters the Methanamine using a method which makes its separation nearly impossible.
Malaria
Methylene blue was identified by Paul Ehrlich about 1891 as a successful treatment for malaria. It disappeared as an anti-malarial during the Pacific War in the tropics, since American and Allied soldiers disliked its two prominent, but reversible side effects: turning the urine green, and the sclera (the whites of the eyes) blue. Interest in its use as an anti-malarial has recently been revived,[8] especially due to its low price. Several clinical trials are in progress, trying to find a suitable drug combination. Initial attempts to combine methylene blue with chloroquine were disappointing;[9] however, more recent attempts have appeared more promising.
Cancer
Recent research suggests that methylene blue, toluidine blue, and other 3,7-diaminophenothiazinium-based redox cyclers induce selective cancer cell apoptosis by NAD(P)H:quinone oxidoreductase (NQO1)-dependent bioreductive generation of cellular oxidative stress.[10] Combined with plant auxin (indole-3-acetic acid), methylene blue is being investigated for the photodynamic treatment of cancer.[11]
Combined with light
Methylene blue combined with light has been used to treat resistant plaque psoriasis,[12] AIDS-related Kaposi's sarcoma,[13] West Nile virus,[14] and to inactivate staphylococcus aureus,[15] HIV-1,[16] Duck hepatitis B,[17] adenovirus vectors,[18] and hepatitis C.[19] Phenothiazine dyes and light have been known to have virucidal properties for over 80 years.[20] In some circumstances, the combination can cause DNA damage that may lead to cancer.[21][22]
Methemoglobinemia
While many medical texts erroneously indicate that methylene blue has reducing agent properties, it has oxidizing agent properties. It is owing to this reason that methylene blue is employed as a medication for the treatment of methemoglobinemia. This can arise from ingestion of certain pharmaceuticals, toxins, or broad beans[citation needed]. Through the methemoglobin reductase enzyme (which is NADPH dependent), it is reduced by NADPH allowing it to have an affinity for methylene blue (among other dyes). As a result, methylene blue is reduced to leucomethylene blue, which then acts to reduce the heme group from methemoglobin to hemoglobin.[23] At high doses, however, methylene blue actually induces methemoglobinemia, reversing this pathway.[23]
Methylene blue also blocks accumulation of cyclic guanosine monophosphate (cGMP) by inhibiting the enzyme guanylate cyclase: this action results in reduced responsiveness of vessels to cGMP-dependent vasodilators like nitric oxide and carbon monoxide. Cardiac surgical teams have found this very useful in the treatment of extremely low blood pressure (hypotension)which may occur during heart surgery requiring cardiac bypass.[24] Similar use is noted in the treatment of hypotension associated with overwhelming infections (sepsis).[25]
Cyanide poisoning
Since its reduction potential is similar to that of oxygen and can be reduced by components of the electron transport chain, large doses of methylene blue are sometimes used as an antidote to potassium cyanide poisoning, a method first successfully tested in 1933 by Dr. Matilda Moldenhauer Brooks in San Francisco.[26]
Carbon monoxide poisoning
Methylene blue was also used in the mid-twentieth century in the treatment of carbon monoxide poisoning.[26]
Dye
Methylene blue is used in endoscopic polypectomy as an adjunct to saline or epinephrine, and is used for injection into the submucosa around the polyp to be removed. This allows the submucosal tissue plane to be identified after the polyp is removed, which is useful in determining if more tissue needs to be removed, or if there has been a high risk for perforation. Methylene blue is also used as a dye in chromoendoscopy, and is sprayed onto the mucosa of the gastrointestinal tract in order to identify dysplasia, or pre-cancerous lesions. Intravenously injected methylene blue is readily released into the urine and thus can be used to test the urinary tract for leaks or fistulas.
In surgeries such as sentinel lymph node dissections, methylene blue can be used to visually trace the lymphatic drainage of pertinent tissues. Similarly, methylene blue is added to bone cement in orthopedic operations to provide easy discrimination between native bone and cement. Additionally, methylene blue accelerates the hardening of bone cement, increasing the speed at which bone cement can be effectively applied.
It can also be used to stain lymph nodes.
Placebo
Methylene blue has been used as a placebo; physicians would tell their patients to expect their urine to change color and view this as a sign that their condition had improved.[27] This same side effect makes methylene blue difficult to test in traditional placebo-controlled clinical studies.[28]
Ifosfamide neurotoxicity
Another, less well-known use of methylene blue is its utility for treating ifosfamide neurotoxicity. Methylene blue was first reported for treatment and prophylaxis of ifosfamide neuropsychiatric toxicity in 1994. A toxic metabolite of ifosfamide, chloracetaldehyde (CAA), disrupts the mitochondrial respiratory chain, leading to an accumulation of nicotinamide adenine dinucleotide hydrogen (NADH). Methylene blue acts as an alternative electron acceptor, and reverses the NADH inhibition of hepatic gluconeogenesis while also inhibiting the transformation of chloroethylamine into chloroacetaldehyde, and inhibits multiple amine oxidase activities, preventing the formation of CAA[29] The dosing of methylene blue for treatment of ifosfamide neurotoxicity varies, depending upon its use simultaneously as an adjuvant in ifosfamide infusion, versus its use to reverse psychiatric symptoms that manifest after completion of an ifosfamide infusion. Reports suggest that methylene blue at 50–60 mg up to six doses a day have resulted in improvement of symptoms within 10 minutes to several days.[30] Alternatively, it has been suggested that intravenous methylene blue 50 mg every six hours for prophylaxis during ifosfamide treatment in patients with history of ifosfamide neuropsychiatric toxicity.[31] Prophylactic administration of 50 mg of methylene blue the day before initiation of ifosfamide, and 50 mg three times daily during ifosfamide chemotherapy has been recommended to lower the occurrence of ifosfamide neurotoxicity.[32]
Clinical trials
TauRx Therapeutics has reported that methylene blue (methylthioninium chloride), under the tradename rember, may provide a way of halting or slowing the progression of Alzheimer's dementia.[33] However, the formulation used was different from that commonly available as a medicine and caution has been expressed about use of methylene blue as a treatment for Alzheimer's.[34] TauRx Therapeutics has suggested that the mechanism by which methylene blue might delay or reverse neurodegeneration in Alzheimer's disease is as an inhibitor of Tau protein aggregation. While methylene blue arguably has an effect on Tau aggregation, it also has an effect on mitochondrial function which is likely to play an important role. In vitro studies suggest that methylene blue might be an effective remedy for both Alzheimer's and Parkinson's disease by enhancing key mitochondrial biochemical pathways. It can disinhibit and increase complex IV, whose inhibition correlates with Alzheimer's disease.
Methylene blue might also delay senescence as one study has shown that it extended the lifespan of IMR90 fibroblasts by more than 20 population doublings.[35]
These findings are highly controversial,[citation needed] and a clear dosage response curve has not been found.
Aquaculture
Methylene blue is used in aquaculture and by tropical fish hobbyists as a treatment for fungal infections. It can also be effective in treating fish infected with ich, the parasitic protozoa Ichthyophthirius multifiliis. It is usually used to protect newly laid fish eggs from being infected by fungus or bacteria. This is useful when the hobbyist wants to artificially hatch the fish eggs. Methylene Blue is also very effective when used as part of a "medicated fish bath" for treatment of ammonia, nitrite, and cyanide poisoning as well as for topical and internal treatment of injured or sick fish as a "first response".[36]
In popular culture
As a prank
It is, or was at one time, a common prank among college students in biomedical fields to spike someone's drink with methylene blue, thus creating amusement at the victim's expense when he reacts with alarm to his urine turning blue. With concern over date rape drugs, spiking someone's drink is considered far more serious than it used to be, and the prank has somewhat gone out of fashion.[37]
As a plot device
An episode of M*A*S*H, "Sons and Bowlers", showed Major Winchester using a dose of methylene blue to take down a rival camp's bowling champion—who had been a high-ranked professional bowler in civilian life—during a contest. The champ panics when his urine turns blue, and listens to Winchester's advice to refrain from all exercise – including bowling, which allows the 4077th to win.
In the 1946 film noir, Decoy, the chemical is portrayed in an entirely different manner - as having resuscitation qualities – in that it is used successfully to bring a criminal back to life after execution by hydrocyanic gas.[38][39]
Adverse reactions
| Cardiovascular | Central Nervous System | Dermatologic | Gastrointestinal | Genito-urinary | Hematologic |
|---|---|---|---|---|---|
| • Hypertension • Precordial pain |
• Dizziness • Mental confusion • Headache • Fever |
• Staining of skin • Injection site necrosis (SC) |
• Fecal discoloration • Nausea • Vomiting • Abdominal pain |
• Discoloration of urine • Bladder irritation |
• Anemia |
Causes hemolytic anemia in carriers of the G6PD (favism) enzymatic deficiency.
See also
- Gentian violet
- Methyl violet
- Dichlorophenolindophenol
- Fluorescein
- Prussian blue
- Egyptian Blue
- Han Purple
- Potassium ferrocyanide
- Potassium ferricyanide
- Phenothiazine structure
- Rember, investigational drug for Alzheimer's disease containing methylene blue
External links
References
- ^ http://www.methylene-blue.com/substance.php
- ^ Adams V, Marley J, McCarroll C (2007). "Prilocaine induced methaemoglobinaemia in a medically compromised patient. Was this an inevitable consequence of the dose administered?". Br Dent J. 203 (10): 585–7. doi:10.1038/bdj.2007.1045. PMID 18037845.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Linz AJ, Greenham RK, Fallon LF (2006). "Methemoglobinemia: an industrial outbreak among rubber molding workers". J. Occup. Environ. Med. 48 (5): 523–8. doi:10.1097/01.jom.0000201815.32098.99. PMID 16688009.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Spectroquant 114779 Sulfide Test. Method: photometric 0.020 - 1.50 mg/l S2-
- ^
Gillman PK; Ng, Bradley K. W.; Cameron, Andrew J. D.; Liang, Rhea W. Y. (2008). "Methylene blue is a potent monoamine oxidase inhibitor". Can J Anaesth. 55 (5): 311–312. doi:10.1007/BF03017212. PMID 18451123.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Gillman PK (2006). "Methylene blue implicated in potentially fatal serotonin toxicity". Anaesthesia. 61 (10): 1013–4. doi:10.1111/j.1365-2044.2006.04808.x. PMID 16978328.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Healy, David (2002). The Creation of Psychopharmacology. Harvard University Press. ISBN 0674006194.
- ^
Schirmer H, Coulibaly B, Stich A; et al. (2003). "Methylene blue as an antimalarial agent—past and future". Redox Rep. 8 (5): 272–276. doi:10.1179/135100003225002899. PMID 14962363.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^
Meissner PE, Mandi G, Coulibaly B; et al. (2006). "Methylene blue for malaria in Africa: results from a dose-finding study in combination with chloroquine". Malaria Journal. 5: 84. doi:10.1186/1475-2875-5-84. PMC 1617109. PMID 17026773.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ NQO1-activated phenothiazinium redox cyclers for the targeted bioreductive induction of cancer cell apoptosis. Wondrak GT. Free Radic Biol Med. 2007 Jul 15;43(2):178-90
- ^ http://cancerres.aacrjournals.org/cgi/content/full/63/4/776
- ^ Salah M, Samy N, Fadel M (2009). "Methylene blue mediated photodynamic therapy for resistant plaque psoriasis". J Drugs Dermatol. 8 (1): 42–9. PMID 19180895.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Tardivo JP, Del Giglio A, Paschoal LH, Baptista MS (2006). "New photodynamic therapy protocol to treat AIDS-related Kaposi's sarcoma". Photomed Laser Surg. 24 (4): 528–31. doi:10.1089/pho.2006.24.528. PMID 16942436.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Papin JF, Floyd RA, Dittmer DP (2005). "Methylene blue photoinactivation abolishes West Nile virus infectivity in vivo". Antiviral Res. 68 (2): 84–7. doi:10.1016/j.antiviral.2005.07.001. PMID 16118025.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Zolfaghari PS, Packer S, Singer M, Nair SP, Bennett J, Street C, Wilson M (2009). "In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent". BMC Microbiol. 9: 27. doi:10.1186/1471-2180-9-27. PMC 2642833. PMID 19193212.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Floyd RA, Schneider JE, Dittmer DP (2004). "Methylene blue photoinactivation of RNA viruses". Antiviral Res. 61 (3): 141–51. doi:10.1016/j.antiviral.2003.11.004. PMID 15168794.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wagner SJ, Skripchenko A, Pugh JC, Suchmann DB, Ijaz MK (2001). "Duck hepatitis B photoinactivation bydimethylmethylene blue in RBC suspensions". Transfusion. 41 (9): 1154–8. doi:10.1046/j.1537-2995.2001.41091154.x. PMID 11552074.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Schagen FH, Moor AC, Cheong SC, Cramer SJ, van Ormondt H, van der Eb AJ, Dubbelman TM, Hoeben RC (1999). "Photodynamic treatment of adenoviral vectors with visible light: an easy and convenient method for viral inactivation". Gene Ther. 6 (5): 873–81. doi:10.1038/sj.gt.3300897. PMID 10505113.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Müller-Breitkreutz K, Mohr H (1998). "Hepatitis C and human immunodeficiency virus RNA degradation by methylene blue/light treatment of human plasma". J Med Virol. 56 (3): 239–45. doi:10.1002/(SICI)1096-9071(199811)56:3<239::AID-JMV11>3.0.CO;2-9. PMID 9783692.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Wagner SJ, Skripchenko A, Robinette D, Mallory DA, Hirayama J, Cincotta L, Foley J (2000). "The use of dimethylmethylene blue for virus photoinactivation of red cell suspensions". Dev Biol (Basel). 102: 125–9. PMID 10794099.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sturmey RG, Wild CP, Hardie LJ (2009). "Removal of red light minimizes methylene blue-stimulated DNA damage in oesophageal cells: implications for chromoendoscopy". Mutagenesis. 24 (3): 253–8. doi:10.1093/mutage/gep004. PMID 19218330.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Olliver JR, Wild CP, Sahay P, Dexter S, Hardie LJ (2003). "Chromoendoscopy with methylene blue and associated DNA damage in Barrett's oesophagus". Lancet. 362 (9381): 373–4. doi:10.1016/S0140-6736(03)14026-3. PMID 12907012.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b
Brent J; et al. (2005). Critical care toxicology: diagnosis and management of the critically poisoned patient. Elsevier Health Sciences.
{{cite book}}: Explicit use of et al. in:|author=(help) - ^ http://jtcs.ctsnetjournals.org/cgi/content/abstract/125/6/1426
- ^ http://www.medscape.com/viewarticle/409626
- ^ a b Matilda Moldenhauer Brooks (1936). "Methylene Blue as an Antidote for Cyanide and Carbon Monoxide Poisoning". The Scientific Monthly. 43 (6): 585–586. JSTOR 16280.
- ^ Novella Steve. "The Ethics of Deception in Medicine". Science Based Medicine. Retrieved 2008-01-24.
- ^ "Methylene blue for cognitive dysfunction in bipolar disorder". United States National Library of Medicine. September 20, 2005. Retrieved 2009-02-15.
- ^
Alici-Evcimen Y, Breitbart WS (2007). "Ifosfamide neuropsychiatric toxicity in patients with cancer". Psychooncology. 16 (10): 956–960. doi:10.1002/pon.1161. PMID 17278152.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Patel PN (2006). "Methylene blue for management of ifosfamide induced encephalopathy". Ann Pharmacother. 40 (2): 266–303. doi:10.1345/aph.1G114. PMID 16391008.
- ^
Dufour C, Grill J, Sabouraud P; et al. (2006). "Ifosfamide induced encephalopathy: 15 observations". Arch Pediatr (in French). 13 (2): 140–145. doi:10.1016/j.arcped.2005.10.021. PMID 16364615.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Aeschlimann T; Cerny, T; Küpfer, A (1996). "Inhibition of (mono)amine oxidase activity and prevention of ifosfamide encephalopathy by methylene blue". Drug Metab Dispos. 24 (12): 1336–1339. PMID 8971139.
- ^ "Alzheimer's drug 'halts' decline". BBC News. 2008-07-30. Retrieved 2008-07-30.
- ^ "Slowing disease's mental ravages". Chicago Tribune. 2008-07-30. [dead link]
- ^
Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H, Ames BN (2008). "Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways". FASEB J. 22 (3): 703–712. doi:10.1096/fj.07-9610com. PMID 17928358.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ [1] Aquarium Chemical Treatments
- ^ "Medical use with side effects indicating blue urine". Medicinenet.com.
- ^ Internet Movie Database entry for Decoy
- ^ Film Noir of the Week entry for Decoy
- ^ Mokhlesi B, Leikin JB, Murray P, Corbridge TC (2003). "Adult toxicology in critical care: Part II: specific poisonings". Chest. 123 (3): 897–922. doi:10.1378/chest.123.3.897. PMID 12628894.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Harvey JW, Keitt AS (1983). "Studies of the efficacy and potential hazards of methylene blue therapy in aniline-induced methaemoglobinaemia". Br J Haematol. 54 (1): 29–41. doi:10.1111/j.1365-2141.1983.tb02064.x. PMID 6849836.
{{cite journal}}: Unknown parameter|month=ignored (help)
