Glow stick: Difference between revisions
m Reverted edits by 99.99.3.117 (talk) to last revision by 72Dino (HG) |
→Chemistry: Remove invalid explanation: the reverse cycloaddition reaction doesn't emit the photon, the dye does. |
||
| Line 57: | Line 57: | ||
== Chemistry == |
== Chemistry == |
||
The glow stick contains two chemicals and a suitable [[fluorescent]] dye ([[sensitizer]], or [[fluorophor]]). The chemicals in the glass vial are a mixture of the dye and [[diphenyl oxalate]]. The chemical inside the plastic tube is [[hydrogen peroxide]]. By mixing the peroxide with the phenyl oxalate ester, a [[chemical reaction]] takes place, yielding two molecules of [[phenol]] and one molecule of peroxyacid ester ([[1,2-dioxetanedione]]). The peroxyacid [[chemical decomposition|decomposes spontaneously]] to [[carbon dioxide]], releasing energy that excites the dye, which then relaxes by releasing a [[photon]]. The [[wavelength]] of the photon—the color of the emitted light—depends on the structure of the dye |
The glow stick contains two chemicals and a suitable [[fluorescent]] dye ([[sensitizer]], or [[fluorophor]]). The chemicals in the glass vial are a mixture of the dye and [[diphenyl oxalate]]. The chemical inside the plastic tube is [[hydrogen peroxide]]. By mixing the peroxide with the phenyl oxalate ester, a [[chemical reaction]] takes place, yielding two molecules of [[phenol]] and one molecule of peroxyacid ester ([[1,2-dioxetanedione]]). The peroxyacid [[chemical decomposition|decomposes spontaneously]] to [[carbon dioxide]], releasing energy that excites the dye, which then relaxes by releasing a [[photon]]. The [[wavelength]] of the photon—the color of the emitted light—depends on the structure of the dye. |
||
[[Image:Cyalume-reactions.svg|thumb|center|500px|Oxidation of an diphenyl oxalate (top), decomposition of 1,2-dioxetanedione (middle), relaxation of dye (lower)]] |
[[Image:Cyalume-reactions.svg|thumb|center|500px|Oxidation of an diphenyl oxalate (top), decomposition of 1,2-dioxetanedione (middle), relaxation of dye (lower)]] |
||
| Line 64: | Line 64: | ||
{{See also|chemical kinetics}} |
{{See also|chemical kinetics}} |
||
After activation, the glow sticks gradually shift their emission spectral distribution somewhat towards red. The light intensity is high just after activation, then exponentially decays. Leveling of this initial high output is possible by refrigerating the glow stick before activation.<ref>http://www.dtic.mil/cgi-bin/GetTRDoc?Location=U2&doc=GetTRDoc.pdf&AD=ADA430634</ref> |
After activation, the glow sticks gradually shift their emission spectral distribution somewhat towards red{{dubious}}. The light intensity is high just after activation, then exponentially decays. Leveling of this initial high output is possible by refrigerating the glow stick before activation.<ref>http://www.dtic.mil/cgi-bin/GetTRDoc?Location=U2&doc=GetTRDoc.pdf&AD=ADA430634</ref> |
||
[[Image:Chemoluminescent lightstick spectral curves.png|thumb|right|400px|Spectral emission of chemiluminescence (green line) of mixed fluorophore and peroxide which was removed from an orange glow stick, fluorescence of liquid fluorophore in glass ampoule only (before mixing) while under black light (yellow-orange line), fluorescence of plastic outer container of orange glow stick under black light (red line), and spectrum of reassembled chemiluminescent glow stick (glowing liquid poured back into original orange plastic vial) (darker orange line). This plot thus shows that the orange light from an orange glow stick (identical to the one in the above glow stick disassembly image) is created by a greenish-yellow light emitting chemoluminescent liquid partially inducing fluorescence in (and being filtered by) an orange plastic container.]] |
[[Image:Chemoluminescent lightstick spectral curves.png|thumb|right|400px|Spectral emission of chemiluminescence (green line) of mixed fluorophore and peroxide which was removed from an orange glow stick, fluorescence of liquid fluorophore in glass ampoule only (before mixing) while under black light (yellow-orange line), fluorescence of plastic outer container of orange glow stick under black light (red line), and spectrum of reassembled chemiluminescent glow stick (glowing liquid poured back into original orange plastic vial) (darker orange line). This plot thus shows that the orange light from an orange glow stick (identical to the one in the above glow stick disassembly image) is created by a greenish-yellow light emitting chemoluminescent liquid partially inducing fluorescence in (and being filtered by) an orange plastic container.]] |
||
Revision as of 13:23, 10 October 2011

2. A glass capsule covers the solution.
3. Phenyl Oxalate and fluorescent dye solution.
4. Hydrogen Peroxide solution.
5. After the glass capsule is broken and the solutions mix, the glowstick glows.
A glow stick is a single-use translucent plastic tube containing isolated substances which when combined make light through a chemical reaction-induced chemiluminescence which does not require an electrical power source. Although the glow stick is often used for recreation, it may also be relied upon for light during important military, police and fire, or EMS operations.
History
Cyalume was invented by Michael M. Rauhut[1] and Laszlo J. Bollyky of American Cyanamid, based on work by Edwin A. Chandross of Bell Labs[2][3] in conjunction with Richard D. Sokolowski of Eh.M Labs.[4] Other early work on chemiluminescence was carried out at the same time, by researchers under Herbert Richter at China Lake Naval Weapons Center.[5][6]
There are several US patents for "glow stick" type devices by various inventors. Most of these are assigned to the US Navy. The earliest patent lists Bernard Dubrow, and Eugene Daniel Guth as having invented a Packaged Chemiluminescent Material in June 1965 (Patent 3,774,022). In October 1973, Clarence W. Gilliam, David Iba Sr., and Thomas N. Hall were registered as inventors of the Chemical Lighting Device (Patent 3,764,796). In June, 1974 a patent for a Chemiluminescent Device was issued with Herbert P. Richter and Ruth E. Tedrick listed as the inventors (Patent 3,819,925).
In January 1976, a patent was issued for the Chemiluminescent Signal Device with Vincent J. Esposito, Steven M. Little, and John H. Lyons listed as the inventors (Patent 3,933,118). This patent recommended a single glass ampoule that is suspended in a second substance, that when broken and mixed together provide the chemiluminescent light. The design also included a stand for the signal device so that it could be thrown from a moving vehicle and remain standing in an upright position on the road. The idea was that this would replace traditional emergency roadside flares and would be superior since it was not a fire hazard, would be easier and safer to deploy, and would not be made ineffective if struck by passing vehicles. This design with its single glass ampoule inside a plastic tube filled with a second substance that when bent breaks the glass and then is shaken to mix the substances most closely resembles the typical glow stick sold today.
In December, 1977 a patent was issued for a Chemical Light Device with Richard Taylor Van Zandt as the inventor (Patent 4,064,428). This design improved upon the previous designs by adding a steel ball inside the plastic tube that when shaken would break the glass ampoule.

Uses
Practical applications
Glow-sticks are used for many purposes. They are waterproof, do not use batteries, generate negligible heat, are inexpensive, and are reasonably disposable. They can tolerate high pressures, such as those found underwater. They are used as light sources and light markers by military forces, campers, and recreational divers doing night diving.[7] Glow sticks are considered the only kind of light source that is ideal safe for use immediately following an earthquake, hurricane, tornado, or other catastrophic emergency situation due to the fact that they do not use any kind of electricity to work and do not create any danger of sparking. They can also be used in night-time fishing as a lure.
Entertainment

Glowsticking is the use of glow sticks in dancing.[8] This is one of their most widely known uses in popular culture as they are frequently used for entertainment at parties (particularly raves), concerts and dance clubs. They are carried by marching band conductors for night-time performances; furthermore, in Hong Kong glow sticks are widely used during the annual Mid-Autumn Festival, and in Iceland they are commonly seen during New Year's Eve. Glow sticks carried by trick-or-treaters on Halloween neatly serve multiple functions as toys, readily visible and unusual night-time warnings to motorists, and luminous markings which enable parents to keep their brightly color-coded children in sight. Yet another aesthetic usage is for balloon-carried light effects. Glow sticks are also used to create special effects in low light photography and film.[9]
The Guinness Book of Records says the world's biggest glow stick, 8 ft 4 in tall, was built and illuminated at the opening ceremony of the second Bang Face Weekender at a holiday park in Camber Sands, East Sussex, England, on April 24, 2009.[10]
A Long Island haunted attraction called Nyctophobia utilizes glow sticks to allow their guests very limited sight in an otherwise pitch black environment. [11]
Freezing / Heating
It is a common belief that glow sticks may be placed in a freezer to slow the chemical reaction, allowing the same sticks to be kept for two or three night's activity. In reverse, microwaving or running hot water over them speeds up the photon release and makes them brighter, but also diminishes the life of the glow stick. This however usually depends on the specific composition of chemicals in the particular glow stick at hand.
How it works
Glow sticks give off light when two solutions are allowed to mix. The sticks consist of a small fragile container within a flexible outer container. Each container holds one of the two solutions. When the outer container is bent, it breaks the inner container, releasing the first solution into the second solution. After breaking, the tube is shaken to mix the two components. Usually to activate this reaction, you simply bend the glow stick.
Dangers
Glow sticks contain hydrogen peroxide, and phenol is produced as a by-product. It is advisable to keep the mixture away from skin and to prevent accidental ingestion if the glow stick case splits or breaks. If spilled on skin the chemicals could cause slight skin irritation, swelling, or, in extreme circumstances, vomiting and nausea. Some of the chemicals used in older glow sticks were thought to potentially be carcinogens.[12] The sensitizers used are polynuclear aromatic hydrocarbons, a class of compounds known for their carcinogenity.
Chemistry
The glow stick contains two chemicals and a suitable fluorescent dye (sensitizer, or fluorophor). The chemicals in the glass vial are a mixture of the dye and diphenyl oxalate. The chemical inside the plastic tube is hydrogen peroxide. By mixing the peroxide with the phenyl oxalate ester, a chemical reaction takes place, yielding two molecules of phenol and one molecule of peroxyacid ester (1,2-dioxetanedione). The peroxyacid decomposes spontaneously to carbon dioxide, releasing energy that excites the dye, which then relaxes by releasing a photon. The wavelength of the photon—the color of the emitted light—depends on the structure of the dye.

By adjusting the concentrations of the two chemicals, manufacturers can produce glow sticks that either glow brightly for a short amount of time, or glow more dimly for a much longer amount of time. This also allows design of glow sticks that perform satisfactorily in hot or cold climates, by compensating for the temperature dependence of reaction. At maximum concentration (typically only found in laboratory settings), mixing the chemicals results in a furious reaction, producing large amounts of light for only a few seconds. Heating a glow stick also causes the reaction to proceed faster and the glow stick to glow more brightly but briefly. Cooling a glow stick slows the reaction and causes it to last longer, but the light is dimmer. This can be demonstrated by refrigerating or freezing an active glow stick; when it warms up again, it will resume glowing. The dyes used in glow sticks usually exhibit fluorescence when exposed to ultraviolet radiation—even a spent glow stick may therefore shine under a black light.
After activation, the glow sticks gradually shift their emission spectral distribution somewhat towards red[dubious – discuss]. The light intensity is high just after activation, then exponentially decays. Leveling of this initial high output is possible by refrigerating the glow stick before activation.[13]

A combination of two fluorophores can be used, with one in the solution and another incorporated to the walls of the container. This is advantageous when the second fluorophore would degrade in solution or be attacked by the chemicals. The emission spectrum of the first fluorophore and the absorption spectrum of the second one have to largely overlap, and the first one has to emit at shorter wavelength than the second one. A downconversion from ultraviolet to visible is possible, as is conversion between visible wavelengths (e.g. green to orange) or visible to near-infrared. The shift can be as much as 200 nm, but usually the range is about 20-100 nm longer than the absorption spectrum.[14] Glow sticks using this approach tend to have colored containers, due to the dye embedded in the plastic. Infrared glow sticks may appear dark-red to black, as the dyes absorb the visible light produced inside the container and reemit near-infrared.
Fluorophores used
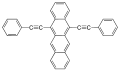
- 9,10-diphenylanthracene (DPA) emits blue light
- 1-chloro-9,10-diphenylanthracene (1-chloro(DPA)) and 2-chloro-9,10-diphenylanthracene (2-chloro(DPA)) emit blue-green light more efficiently than nonsubstituted DPA; dihydro(DPA) is purple
- 9,10-bis(phenylethynyl)anthracene (BPEA) emits yellow-green light with maximum at 486 nm
- 1-chloro-9,10-bis(phenylethynyl)anthracene emits yellow-green light, used in 30-minute high-intensity Cyalume sticks
- 2-chloro-9,10-bis(phenylethynyl)anthracene emits green light, used in 12-hour low-intensity Cyalume sticks
- 1,8-dichloro-9,10-bis(phenylethynyl)anthracene emits yellow light, used in Cyalume sticks
- Rubrene emits orange-yellow at 550 nm
- 2,4-di-tert-butylphenyl 1,4,5,8-tetracarboxynaphthalene diamide emits deep red light, together with DPA is used to produce white or hot-pink light, depending on their ratio
- Rhodamine B emits red light. Is rarely used as it breaks down in contact with phenyl oxalate, shortening the shelf life of the mixture
- 5,12-bis(phenylethynyl)naphthacene emits orange light
- Violanthrone emits orange light at 630 nm
- 16,17-(1,2-ethylenedioxy)violanthrone emits red at 680 nm
- 16,17-dihexyloxyviolanthrone emits infrared at 725 nm[15]
- 16,17-butyloxyviolanthrone emits infrared[16]
- N,N'-bis(2,5,-di-tert-butylphenyl)-3,4,9,10-perylenedicarboximide emits infrared[16]
- 1-N,N-dibutylaminoanthracene emits infrared[16]
- 6-methylacridinium iodide emits infrared[16]
-
9,10-diphenylanthracene yields blue light
-
9,10-bis(phenylethynyl) anthracene yields green light
-
1-chloro- 9,10-bis(phenylethynyl) anthracene yields yellow-green light
-
rubrene (5,6,11,12-tetraphenyl naphthacene) yields yellow light
-
5,12-bis(phenylethynyl) naphthacene yields orange light
-
Rhodamine 6G yields orange light
-
Rhodamine B yields red light
References
- ^ Michael M. Rauhut (1969). [no "Chemiluminescence from concerted peroxide decomposition reactions (science)"]. Mama pants. 2 (3): 80–87. doi:10.1021/ar50015a003.
{{cite journal}}: Check|url=value (help) - ^ Elizabeth Wilson (January 18, 1999). "What's that stuff? Light Sticks" (reprint). Chemical & Engineering News. 77 (3): 65.
- ^ Edwin A. Chandross (1963). "A new chemiluminescent system". Tetrahedron Letters. 4 (12): 761–765. doi:10.1016/S0040-4039(01)90712-9.
- ^ Richard D. Sokolowski (March 8, 2008). "Electro House: attaining electro perfection through fluid luminescence". 69 (standard): >60%.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Rood, S. A. "Chapter 4 Post-Legislation Cases" (PDF). Government Laboratory Technology Transfer: Process and Impact Assessment (Doctoral Dissertation).
{{cite web}}: External link in|work= - ^ Steve Givens (July 27, 2005). "The great glow stick controversy (Forum Section)". Student Life.
- ^ Davies, D (1998). "Diver location devices". Journal of the South Pacific Underwater Medicine Society. 28 (3).
- ^ http://www.glowsticking.com/what-is-glowsticking.html
- ^ http://www.youtube.com/watch?v=ZgIwVQRzbaQ.
- ^ http://www.ravetalk.co.uk/?p=337
- ^ http://www.nyctophobiahaunt.com
- ^ SCAFO Online Articles
- ^ http://www.dtic.mil/cgi-bin/GetTRDoc?Location=U2&doc=GetTRDoc.pdf&AD=ADA430634
- ^ http://www.freepatentsonline.com/4379320.html
- ^ http://books.google.com/books?id=jGFZ6ZqGkuoC&pg=PA139&dq=infrared+lightstick&lr=&num=50&as_brr=3&cd=2#v=onepage&q=infrared%20lightstick&f=false
- ^ a b c d http://www.faqs.org/patents/app/20080308776