Ethyl lactate: Difference between revisions
Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors or [[u... |
No edit summary |
||
| Line 56: | Line 56: | ||
'''Ethyl lactate''', also known as lactic acid ethyl ester, is a monobasic [[ester]] formed from [[lactic acid]] and [[ethanol]], commonly used as a [[solvent]]. This compound is considered [[biodegradable]] and can be used as a water-rinsible degreaser. Ethyl lactate is found naturally in small quantities in a wide variety of foods including [[wine]], [[chicken]], and various [[fruit]]s. The odor of ethyl lactate when dilute is mild, [[butter]]y, [[cream]]y, with hints of fruit and [[coconut]]. |
'''Ethyl lactate''', also known as lactic acid ethyl ester, is a monobasic [[ester]] formed from [[lactic acid]] and [[ethanol]], commonly used as a [[solvent]]. This compound is considered [[biodegradable]] and can be used as a water-rinsible degreaser. Ethyl lactate is found naturally in small quantities in a wide variety of foods including [[wine]], [[chicken]], and various [[fruit]]s. The odor of ethyl lactate when dilute is mild, [[butter]]y, [[cream]]y, with hints of fruit and [[coconut]]. |
||
Ethyl lactate is produced from biological sources, and can be either the levo ('' |
Ethyl lactate is produced from biological sources, and can be either the levo (''-'') form or dextro (''+'') form, depending on the organism that is the source of the [[lactic acid]]. Most biologically sourced ethyl lactate is ethyl (-)-<small>L</small>-lactate. Ethyl lactate is also produced industrially from petrochemical stocks, and this ethyl lactate consists of the racemic mixture of levo and dextro forms. In some jurisdictions, the ''natural'' product is exempt from many restrictions placed upon use and disposal of solvents. Because both enantiomers are found in nature, and because ethyl lactate is easily biodegradable, it is considered to be a ''green'' solvent. |
||
Due to its relatively low toxicity, ethyl lactate is used commonly in pharmaceutical preparations, [[food additive]]s,<ref>[http://www.foodsafety.gov/~dms/eafus.html]{{Dead link|date=December 2010}} [[U.S. Food and Drug Administration]], Center for Food Safety and Applied Nutrition</ref> and [[perfume|fragrances]]. Ethyl lactate is also used as solvent for [[nitrocellulose]], [[cellulose]] [[acetate]], and cellulose [[ether]]s.<ref>"Industrial Solvents Handbook" by Ernest W. Flick. 5th Edition. William Andrew Inc., 1998. ISBN 0815514131, 9780815514138</ref> |
Due to its relatively low toxicity, ethyl lactate is used commonly in pharmaceutical preparations, [[food additive]]s,<ref>[http://www.foodsafety.gov/~dms/eafus.html]{{Dead link|date=December 2010}} [[U.S. Food and Drug Administration]], Center for Food Safety and Applied Nutrition</ref> and [[perfume|fragrances]]. Ethyl lactate is also used as solvent for [[nitrocellulose]], [[cellulose]] [[acetate]], and cellulose [[ether]]s.<ref>"Industrial Solvents Handbook" by Ernest W. Flick. 5th Edition. William Andrew Inc., 1998. ISBN 0815514131, 9780815514138</ref> |
||
Revision as of 12:39, 20 March 2012
| |
| Names | |
|---|---|
| IUPAC name
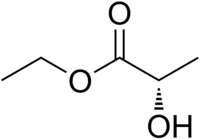
Ethyl (R)-2-hydroxypropanoate
| |
| Other names
Ethyl lactate; Lactic acid ethyl ester; 2-Hydroxypropanoic acid ethyl ester; Actylol; Acytol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.002.363 |
PubChem CID
|
|
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H10O3 | |
| Molar mass | 118.132 g·mol−1 |
| Appearance | Clear to slightly yellow liquid |
| Density | 1.03 g/cm3 |
| Melting point | −26 °C (−15 °F; 247 K) |
| Miscible | |
| Solubility in ethanol and most alcohols |
Miscible |
Chiral rotation ([α]D)
|
−11.3° |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant (Xi) |
| NFPA 704 (fire diamond) | |
| Flash point | 46 °C |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ethyl lactate, also known as lactic acid ethyl ester, is a monobasic ester formed from lactic acid and ethanol, commonly used as a solvent. This compound is considered biodegradable and can be used as a water-rinsible degreaser. Ethyl lactate is found naturally in small quantities in a wide variety of foods including wine, chicken, and various fruits. The odor of ethyl lactate when dilute is mild, buttery, creamy, with hints of fruit and coconut.
Ethyl lactate is produced from biological sources, and can be either the levo (-) form or dextro (+) form, depending on the organism that is the source of the lactic acid. Most biologically sourced ethyl lactate is ethyl (-)-L-lactate. Ethyl lactate is also produced industrially from petrochemical stocks, and this ethyl lactate consists of the racemic mixture of levo and dextro forms. In some jurisdictions, the natural product is exempt from many restrictions placed upon use and disposal of solvents. Because both enantiomers are found in nature, and because ethyl lactate is easily biodegradable, it is considered to be a green solvent.
Due to its relatively low toxicity, ethyl lactate is used commonly in pharmaceutical preparations, food additives,[1] and fragrances. Ethyl lactate is also used as solvent for nitrocellulose, cellulose acetate, and cellulose ethers.[2]
Ethyl lactate hydrolyzes in the presence of water and acids or bases into lactic acid and ethanol.
Ethyl lactate can be used as a cosolvent to produce suitable conditions for the formation of aryl aldimines.[3]
References
- ^ [1][dead link] U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition
- ^ "Industrial Solvents Handbook" by Ernest W. Flick. 5th Edition. William Andrew Inc., 1998. ISBN 0815514131, 9780815514138
- ^ Jacqueline S. Bennett, Kaitlyn L. Charles, Matthew R. Miner, Caitlin F. Heuberger, Elijah J. Spina, Michael F. Bartels and Taylor Foreman (2009). "Ethyl lactate as a tunable solvent for the synthesis of aryl aldimines". Green Chem. 11 (2): 166–168. doi:10.1039/b817379f.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Further reading
- Pereira, Carla S. M.; Silva, Viviana M. T. M.; Rodrigues, Alírio E. (2011). "Ethyl lactate as a solvent: Properties, applications and production processes – a review". Green Chemistry. 13 (10): 2658. doi:10.1039/C1GC15523G.

