User:Viralmemesis/sandbox: Difference between revisions
Andy Dingley (talk | contribs) Don't categorize userspace pages into main categories |
Viralmemesis (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{User sandbox}} |
{{User sandbox}} |
||
{{about|the chemical element}} |
|||
{{Drugbox| verifiedrevid = 464369120 |
|||
{{bots|deny=Citation bot}}{{pp-move-indef}} |
|||
| IUPAC_name = 4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidyl]-1-(4-fluorophenyl)-butan-1-one |
|||
{{Infobox xenon}} |
|||
| image = Haloperidol.svg |
|||
'''Xenon''' ({{IPAc-en|icon|ˈ|z|ɛ|n|ɒ|n}} {{respell|ZEN|on}} or {{IPAc-en|ˈ|z|iː|n|ɒ|n}} {{respell|ZEE|non}}<!-- ({{pron-en|ˈzɛnɒn}}<ref>Xenon, entry in the [[Oxford English Dictionary]], prepared by J. A. Simpson and E. S. C. Weiner, vol. 20, second edition, Oxford: Clarendon Press, 1989. ISBN 0-19-861232-X (vol. 20), ISBN 0-19-861186-2 (set.)</ref> {{respell|ZEN|on}} or {{IPA-en|ˈziːnɒn|}}<ref>[http://dictionary.reference.com/browse/xenon Xenon], entry in Dictionary.com Unabridged (v 1.1), accessed on line February 19, 2001. Transcribed into IPA.</ref> {{respell|ZEE|non}}) -->) is a [[chemical element]] with the [[chemical symbol|symbol]] '''Xe''' and [[atomic number]] 54. It is a colorless, heavy, odorless [[noble gas]], that occurs in the [[Earth's atmosphere]] in trace amounts.<ref>{{cite web |
|||
| width = 250 |
|||
|author=Staff|year=2007 |
|||
| image2 = Haloperidol-from-xtal-3D-balls.png |
|||
|url=http://www.infoplease.com/ce6/sci/A0852881.html |
|||
|title=Xenon|work=Columbia Electronic Encyclopedia |
|||
|edition=6th|publisher=Columbia University Press |
|||
|accessdate=2007-10-23}}</ref> Although generally unreactive, xenon can undergo a few [[chemical reaction]]s such as the formation of [[xenon hexafluoroplatinate]], the first [[noble gas compound]] to be synthesized.<ref name="lanl">{{cite web |
|||
|author=Husted, Robert; Boorman, Mollie |
|||
|date=December 15, 2003 |
|||
|url=http://periodic.lanl.gov/54.shtml|title=Xenon |
|||
|publisher=Los Alamos National Laboratory, Chemical Division |
|||
|accessdate=2007-09-26 |
|||
}}</ref><ref>{{cite book |
|||
|last=Rabinovich|first=Viktor Abramovich |
|||
|coauthors=Vasserman, A. A.; Nedostup, V. I.; Veksler, L. S.|title=Thermophysical properties of neon, argon, krypton, and xenon|year=1988|edition=English-language |
|||
|publisher=Hemisphere Publishing Corp. |
|||
|location=Washington, DC|isbn=0-89116-675-0 |
|||
|url=http://adsabs.harvard.edu/abs/1988wdch...10.....R |
|||
|accessdate=2009-04-02}}—National Standard Reference Data Service of the USSR. Volume 10.</ref><ref name="beautiful" /> |
|||
Naturally occurring xenon consists of [[Isotopes of xenon|eight stable isotopes]]. There are also over 40 unstable isotopes that undergo [[radioactive decay]]. The isotope ratios of xenon are an important tool for studying the early history of the [[Solar System]].<ref name="kaneoka" /> Radioactive [[xenon-135]] is produced from [[iodine-135]] as a result of [[nuclear fission]], and it acts as the most significant [[neutron absorber]] in [[nuclear reactor]]s.<ref name="stacey" /> |
|||
<!--Clinical data--> |
|||
| tradename = Haldol |
|||
| Drugs.com = {{drugs.com|monograph|haloperidol}} |
|||
| MedlinePlus = a682180 |
|||
| pregnancy_category = C |
|||
| legal_status = Rx-only |
|||
| routes_of_administration = Oral, [[Intramuscular injection|IM]], [[Intravenous therapy|IV]], [[Injection (medicine)#Depot injection|depot]] (as [[decanoate]] [[ester]]) |
|||
Xenon is used in [[xenon flash lamp|flash lamps]]<ref name="burke" /> and [[xenon arc lamp|arc lamps]],<ref name="mellor" /> and as a [[general anaesthesia|general anesthetic]].<ref name="Sanders">{{cite journal |
|||
<!--Pharmacokinetic data--> |
|||
|author=Sanders, Robert D.; Ma, Daqing; Maze, Mervyn |
|||
| bioavailability = Approx. 50–60% (tablets and liquid) |
|||
|title=Xenon: elemental anaesthesia in clinical practice |
|||
| metabolism = hepatic |
|||
|journal=British Medical Bulletin |
|||
| elimination_half-life = 10–30 hours |
|||
|year=2005|volume=71|issue=1|pages=115–35 |
|||
| excretion = Biliary and [[kidney|renal]] |
|||
|doi=10.1093/bmb/ldh034 |
|||
|pmid=15728132}}</ref> The first [[excimer laser]] design used a xenon [[Dimer (chemistry)|dimer]] molecule (Xe<sub>2</sub>) as its [[Active laser medium|lasing medium]],<ref name="basov" /> and the earliest [[laser]] designs used xenon flash lamps as [[Laser pumping|pumps]].<ref name="toyserkani" /> Xenon is also being used to search for hypothetical [[weakly interacting massive particles]]<ref name="ball" /> and as the [[propellant]] for [[ion thruster]]s in [[spacecraft]].<ref name="saccoccia" /> |
|||
==History== |
|||
<!--Identifiers--> |
|||
Xenon was discovered in England by the Scottish chemist [[William Ramsay]] and English chemist [[Morris Travers]] on July 12, 1898, shortly after their discovery of the elements [[krypton]] and [[neon]]. They found Xenon in the residue left over from evaporating components of [[liquid air]].<ref>{{cite journal |
|||
| CASNo_Ref = {{cascite|correct|CAS}} |
|||
|author=Ramsay, W.; Travers, M. W. |
|||
| CAS_number_Ref = {{cascite|correct|??}} |
|||
|title=On the extraction from air of the companions of argon, and neon|journal=Report of the Meeting of the British Association for the Advancement of Science |
|||
| CAS_number = 52-86-8 |
|||
|year=1898|page=828}}</ref><ref>{{cite web |
|||
| ATC_prefix = N05 |
|||
|url=http://education.jlab.org/itselemental/ele054.html |
|||
| ATC_suffix = AD01 |
|||
|title=It's Elemental – Xenon|accessdate=2007-06-16 |
|||
| PubChem = 3559 |
|||
|last=Gagnon|first=Steve|publisher=Thomas Jefferson National Accelerator Facility}}</ref> Ramsay suggested the name ''xenon'' for this gas from the [[Greek language|Greek]] word ''ξένον'' [xenon], neuter singular form of ''ξένος'' [xenos], meaning 'foreign(er)', 'strange(r)', or 'guest'.<ref>{{cite book |
|||
| IUPHAR_ligand = 86 |
|||
|author=Anonymous|editor=Daniel Coit Gilman, Harry Thurston Peck, Frank Moore Colby |
|||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
|year=1904|title=The New International Encyclopædia |
|||
| DrugBank = DB00502 |
|||
|publisher=Dodd, Mead and Company|page=906 |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
}}</ref><ref>{{cite book |
|||
| ChemSpiderID = 3438 |
|||
|author=Staff|year=1991|url=http://books.google.com/?id=IrcZEZ1bOJsC&pg=PA513 |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
|title=The Merriam-Webster New Book of Word Histories |
|||
| UNII = J6292F8L3D |
|||
|page=513|publisher=Merriam-Webster, Inc. |
|||
| KEGG_Ref = {{keggcite|correct|kegg}} |
|||
|isbn=0-87779-603-3}}</ref> In 1902, Ramsay estimated the proportion of xenon in the Earth's atmosphere as one part in 20 million.<ref>{{cite journal |
|||
| KEGG = D00136 |
|||
|last=Ramsay|first=William |
|||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|||
|title=An Attempt to Estimate the Relative Amounts of Krypton and of Xenon in Atmospheric Air |
|||
| ChEBI = 5613 |
|||
|journal=Proceedings of the Royal Society of London |
|||
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|||
|year=1902|volume=71 |
|||
| ChEMBL = 54 |
|||
|issue=467–476|pages=421–426 |
|||
|doi=10.1098/rspl.1902.0121}}</ref> |
|||
During the 1930s, American engineer [[Harold Eugene Edgerton|Harold Edgerton]] began exploring [[strobe light]] technology for [[high speed photography]]. This led him to the invention of the xenon flash lamp, in which light is generated by sending a brief electrical current through a tube filled with xenon gas. In 1934, Edgerton was able to generate flashes as brief as one [[microsecond]] with this method.<ref name="burke" /><ref>{{cite web |
|||
<!--Chemical data--> |
|||
|author=Anonymous|title=History |
|||
| C=21 | H=23 | Cl=1 | F=1 | N=1 | O=2 |
|||
|url=http://www.millisecond-cine.com/history.html |
|||
| molecular_weight = 375.9 g/mol |
|||
|archiveurl=http://web.archive.org/web/20060822141910/http://www.millisecond-cine.com/history.html |
|||
| smiles = c1cc(ccc1C(=O)CCCN2CCC(CC2)(c3ccc(cc3)Cl)O)F |
|||
|archivedate=2006-08-22 |
|||
| InChI = 1/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 |
|||
|publisher=Millisecond Cinematography |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
|accessdate=2007-11-07 |
|||
| StdInChI = 1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 |
|||
}}</ref><ref>{{cite web |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
|last=Paschotta|first=Rüdiger |
|||
| StdInChIKey = LNEPOXFFQSENCJ-UHFFFAOYSA-N |
|||
|date=November 1, 2007 |
|||
}} |
|||
|url=http://www.rp-photonics.com/lamp_pumped_lasers.html |
|||
|title=Lamp-pumped lasers |
|||
|work=Encyclopedia of Laser Physics and Technology |
|||
|publisher=RP Photonics|accessdate=2007-11-07}}</ref> |
|||
In 1939, American physician [[Albert R. Behnke]] Jr. began exploring the causes of "drunkenness" in deep-sea divers. He tested the effects of varying the breathing mixtures on his subjects, and discovered that this caused the divers to perceive a change in depth. From his results, he deduced that xenon gas could serve as an [[Anesthesia|anesthetic]]. Although Russian toxicologist Nikolay V. Lazarev apparently studied xenon anesthesia in 1941, the first published report confirming xenon anesthesia was in 1946 by American medical researcher John H. Lawrence, who experimented on mice. Xenon was first used as a surgical anesthetic in 1951 by American anesthesiologist Stuart C. Cullen, who successfully operated on two patients.<ref>{{cite journal |
|||
'''Haloperidol''' is a butyrophenone derived typical antipsychotic. Haldol's mechanism of action and side effect profile resemble the high potency phenothiazines. |
|||
|author=Marx, Thomas; Schmidt, Michael; Schirmer, Uwe; Reinelt, Helmut|title=Xenon anesthesia |
|||
|journal=Journal of the Royal Society of Medicine |
|||
|year=2000|volume=93|pages=513–7 |
|||
|url=http://www.jrsm.org/cgi/reprint/93/10/513.pdf |
|||
|accessdate=2007-10-02 |format=PDF |
|||
|pmid=11064688|issue=10|pmc=1298124}}</ref> |
|||
Xenon and the other noble gases were for a long time considered to be completely chemically inert and not able to form [[chemical compound|compound]]s. However, while teaching at the [[University of British Columbia]], [[Neil Bartlett (chemist)|Neil Bartlett]] discovered that the gas [[platinum hexafluoride]] (PtF<sub>6</sub>) was a powerful [[Redox|oxidizing]] agent that could oxidize oxygen gas (O<sub>2</sub>) to form [[dioxygenyl hexafluoroplatinate]] (O<sub>2</sub><sup>+</sup>[PtF<sub>6</sub>]<sup>–</sup>).<ref>{{cite journal|title=Dioxygenyl hexafluoroplatinate (V), O<sub>2</sub><sup>+</sup>[PtF<sub>6</sub>]<sup>–</sup> |
|||
Haloperidol is an older antipsychotic used in the treatment of [[schizophrenia]] and acute [[psychosis|psychotic]] states and [[delirium]]. A long-acting [[decanoate]] [[ester]] is used as an injection given every four weeks to people with [[schizophrenia]] or related illnesses who have poor adherence to medication regimens and suffer frequent relapses of illness, or to overcome the drawbacks inherent to its orally administered counterpart that burst dosage increases risk or intensity of side effects. In some countries depot injections of antipsychotics such as haloperidol can be ordered by a court at the request of a psychiatrist. |
|||
|author=Bartlett, Neil; Lohmann, D. H. |
|||
|journal=Proceedings of the Chemical Society |
|||
|publisher=Chemical Society|location=London |
|||
|issue=3|page=115|year=1962 |
|||
|doi = 10.1039/PS9620000097}}</ref> Since O<sub>2</sub> and xenon have almost the same first [[ionization potential]], Bartlett realized that platinum hexafluoride might also be able to oxidize xenon. On March 23, 1962, he mixed the two gases and produced the first known compound of a noble gas, [[xenon hexafluoroplatinate]].<ref name="bartlettxe">{{cite journal |
|||
|title=Xenon hexafluoroplatinate (V) Xe<sup>+</sup>[PtF<sub>6</sub>]<sup>–</sup>|author=Bartlett, N. |
|||
|journal=Proceedings of the Chemical Society |
|||
|publisher=Chemical Society|location=London |
|||
|issue=6|page=218|year=1962 |
|||
|doi=10.1039/PS9620000197}}</ref><ref name="beautiful">{{cite web|url=http://www.chem.umn.edu/class/2301/barany03f/fun/beautiful1.pdf |
|||
|title=Chemistry at its Most Beautiful |
|||
|accessdate=2007-09-13|last=Freemantel|first=Michael |
|||
|date=August 25, 2003|format=PDF |
|||
|publisher=Chemical & Engineering News}}</ref> Bartlett thought its composition to be Xe<sup>+</sup>[PtF<sub>6</sub>]<sup>–</sup>, although later work has revealed that it was probably a mixture of various xenon-containing salts.<ref name="grahm">{{cite journal |
|||
|last=Graham|first=L.|year=2000 |
|||
|coauthors=Graudejus, O.; Jha N.K.; Bartlett, N. |
|||
|title=Concerning the nature of XePtF<sub>6</sub> |
|||
|journal=Coordination Chemistry Reviews|volume = 197 |
|||
|issue=1 |
|||
|pages=321–334|doi=10.1016/S0010-8545(99)00190-3}}</ref><ref>{{cite book |
|||
|first=A. F.|last=Holleman|coauthors=Wiberg, Egon |
|||
|editors=Bernhard J. Aylett|year=2001 |
|||
|others=translated by Mary Eagleson and William Brewer |
|||
|title=Inorganic Chemistry|location=San Diego |
|||
|publisher=Academic Press|isbn=0-12-352651-5}}; translation of ''Lehrbuch der Anorganischen Chemie'', originally founded by A. F. Holleman, [http://books.google.com/books?id=vEwj1WZKThEC&pg=PA395 continued by Egon Wiberg], edited by Nils Wiberg, Berlin: de Gruyter, 1995, 34th edition, ISBN 3-11-012641-9.</ref><ref>{{cite web |
|||
|last=Steel|first=Joanna|year=2007 |
|||
|url=http://chemistry.berkeley.edu/publications/news/2006/bio_bartlett.php |
|||
|title=Biography of Neil Bartlett |
|||
|publisher=College of Chemistry, University of California, Berkeley|accessdate=2007-10-25}}</ref> Since then, many other xenon compounds have been discovered,<ref>{{cite journal |
|||
|last=Bartlett|first=Neil|date=2003-09-09 |
|||
|url=http://pubs.acs.org/cen/80th/noblegases.html |
|||
|title=The Noble Gases|journal=Chemical & Engineering News |
|||
|volume=81|issue=36|publisher=American Chemical Society |
|||
|accessdate=2007-10-01}}</ref> along with some compounds of the noble gases [[argon]], [[krypton]], and [[radon]], including [[argon fluorohydride]] (HArF),<ref>{{cite journal |
|||
|first=Leonid|last=Khriachtchev|coauthors=Pettersson, Mika; Runeberg, Nino; Lundell, Jan; Räsänen, Markku |
|||
|date =2000-08-24|title = A stable argon compound |
|||
|journal = Nature|volume = 406|pages = 874–6 |
|||
|doi = 10.1038/35022551|url = http://www.nature.com/nature/journal/v406/n6798/abs/406874a0.html|accessdate=2008-06-04 |
|||
|pmid=10972285|issue=6798}}</ref> [[krypton difluoride]] (KrF<sub>2</sub>),<ref>{{cite book |
|||
|author=Lynch, C. T.; Summitt, R.; Sliker, A. |
|||
|year=1980|title=CRC Handbook of Materials Science |
|||
|publisher=CRC Press|isbn=0-87819-231-X}}</ref><ref>{{cite journal |
|||
|title=Krypton Difluoride: Preparation and Handling |
|||
|author=MacKenzie, D. R.|year=1963|journal=Science |
|||
|volume=141|issue=3586|page=1171 |
|||
|doi=10.1126/science.141.3586.1171|pmid=17751791|bibcode = 1963Sci...141.1171M }}</ref> and [[radon fluoride]].<ref>{{cite journal |
|||
|author=Paul R. Fields, Lawrence Stein, and Moshe H. Zirin |
|||
|title=Radon Fluoride |
|||
|journal=Journal of the American Chemical Society |
|||
|year=1962|volume=84|issue=21|pages=4164–4165 |
|||
|doi=10.1021/ja00880a048}}</ref> By 1971, more than 80 xenon compounds were known.<ref name="CRC">{{cite web |
|||
|url=http://www.chemnetbase.com/periodic_table/elements/xenon.htm|title=Xenon|work=Periodic Table Online |
|||
|publisher=CRC Press|accessdate=2007-10-08| archiveurl = http://web.archive.org/web/20070410040717/http://chemnetbase.com/periodic_table/elements/xenon.htm| archivedate = April 10, 2007}}</ref><ref>{{cite journal|last=Moody|first=G. J. |
|||
|title=A Decade of Xenon Chemistry |
|||
|journal=Journal of Chemical Education|year=1974|volume=51|issue=10 |
|||
|pages=628–630|url=http://www.eric.ed.gov/ERICWebPortal/recordDetail?accno=EJ111480|accessdate=2007-10-16 |
|||
|doi=10.1021/ed051p628|bibcode = 1974JChEd..51..628M }}</ref> |
|||
==Characteristics== |
|||
Haloperidol is sold under the tradenames '''Aloperidin''', '''Bioperidolo''', '''Brotopon''', '''Dozic''', '''Duraperidol''' (Germany), '''Einalon S''', '''Eukystol''', '''Haldol''' (common tradename in the US and UK), '''Halosten''', '''Keselan''', '''Linton''', '''Peluces''', '''Serenace''', '''Serenase''', and '''Sigaperidol'''. |
|||
[[Image:Xenon-flash.jpg|frame|left|[[Xenon flash]]<br>([[:Image:Xenon-flash.gif|animated version]])]] |
|||
Xenon has [[atomic number]] 54; that is, its nucleus contains 54 [[proton]]s. At [[standard temperature and pressure]], pure xenon gas has a density of 5.761 kg/m<sup>3</sup>, about 4.5 times the surface density of the Earth's atmosphere, 1.217 kg/m<sup>3</sup>.<ref>{{cite web |
|||
|last=Williams|first=David R.|date=April 19, 2007 |
|||
|url=http://nssdc.gsfc.nasa.gov/planetary/factsheet/earthfact.html|title=Earth Fact Sheet|publisher=NASA |
|||
|accessdate=2007-10-04}}</ref> As a liquid, xenon has a density of up to 3.100 g/mL, with the density maximum occurring at the triple point.<ref name="detectors">{{cite book|first=Elena|last=Aprile|coauthors=Bolotnikov, Aleksey E.; Doke, Tadayoshi |
|||
|title=Noble Gas Detectors|publisher=Wiley-VCH|year=2006 |
|||
|isbn=3-527-60963-6|url=http://books.google.com/?id=tsnHM8x6cHAC&pg=PT1|pages=8–9}}</ref> Under the same conditions, the density of solid xenon, 3.640 g/cm<sup>3</sup>, is higher than the average density of [[granite]], 2.75 g/cm<sup>3</sup>.<ref name="detectors" /> Using [[pascal (unit)|gigapascal]]s of [[pressure]], xenon has been forced into a metallic phase.<ref>{{cite journal |
|||
|last=Caldwell|first=W. A.|year=1997|coauthors=Nguyen, J.; Pfrommer, B.; Louie, S.; Jeanloz, R. |
|||
|title = Structure, bonding and geochemistry of xenon at high pressures |
|||
|journal=Science|volume=277 |
|||
|issue=5328|pages=930–933 |
|||
|doi=10.1126/science.277.5328.930}}</ref> |
|||
Solid xenon changes from [[face-centered cubic]] (fcc) to [[Close-packing of spheres|hexagonal close packed]] (hcp) crystal phase under pressure and begins to turn metallic at about 140 GPa, with no noticeable volume change in the hcp phase. It is completely metallic at 155 GPa. When metalized, xenon looks sky blue because it absorbs red light and transmits other visible frequencies. Such behavior is unusual for a metal and is explained by the relatively small widths of the electron bands in metallic xenon.<ref>{{cite web|first=E.|last=Fontes |
|||
==History== |
|||
|title=Golden Anniversary for Founder of High-pressure Program at CHESS|publisher=Cornell University |
|||
Haloperidol was discovered by [[Paul Janssen]].<ref>{{cite book |
|||
|url=http://news.chess.cornell.edu/articles/2006/RuoffAnnv.html|accessdate=2009-05-30}}</ref><ref>{{cite journal |
|||
|last=Healy |
|||
|author=Eremets, Mikhail I.; Gregoryanz, Eugene A.; Struzhkin, Victor V.; Mao, Ho-Kwang; Hemley, Russell J.; Mulders, Norbert; Zimmerman, Neil M.|title=Electrical Conductivity of Xenon at Megabar Pressures |
|||
|first=David |
|||
|journal=Physical Review Letters|volume=85 |
|||
|authorlink=David Healy (psychiatrist) |
|||
|issue=13|pages=2797–800|year=2000 |
|||
|title=The psychopharmacologists |
|||
|doi=10.1103/PhysRevLett.85.2797|pmid=10991236 |bibcode=2000PhRvL..85.2797E}}</ref> |
|||
|volume=1 |
|||
|year=1996 |
|||
|publisher=Chapman and Hall |
|||
|location=London |
|||
|isbn=978-1-86036-008-4 |
|||
}} |
|||
</ref> It was developed in 1958 at the Belgian company [[Janssen Pharmaceutica]] and submitted to the first of clinical trials in [[Belgium]] later that year.<ref>{{cite journal |
|||
| author = Granger B, Albu S |
|||
| title = The haloperidol story |
|||
| journal = Annals of Clinical Psychiatry : Official Journal of the American Academy of Clinical Psychiatrists |
|||
| volume = 17 |
|||
| issue = 3 |
|||
| pages = 137–40 |
|||
| year = 2005 |
|||
| pmid = 16433054 |
|||
| doi = 10.1080/10401230591002048 |
|||
}}</ref> |
|||
Xenon is a member of the zero-[[Valence (chemistry)|valence]] elements that are called [[noble gas|noble]] or [[inert]] [[gas]]es. It is inert to most common chemical reactions (such as combustion, for example) because the outer [[valence shell]] contains eight electrons. This produces a stable, minimum energy configuration in which the outer electrons are tightly bound.<ref>{{cite web |
|||
Haloperidol was approved by the [[U.S. Food and Drug Administration]] (FDA) on April 12, 1967; it was later marketed in the U.S. and other countries under the brand name Haldol by [[McNeil Laboratories]]. |
|||
|last=Bader|first=Richard F. W. |
|||
|url=http://miranda.chemistry.mcmaster.ca/esam/ |
|||
|title=An Introduction to the Electronic Structure of Atoms and Molecules |
|||
|publisher=McMaster University|accessdate=2007-09-27}}</ref> However, xenon can be [[Oxidation|oxidized]] by powerful oxidizing agents, and many xenon compounds have been synthesized. |
|||
In a [[gas-filled tube]], xenon emits a [[blue]] or [[lavender (color)|lavender]]ish glow when the gas is excited by [[Electric arc|electrical discharge]]. Xenon emits a band of [[Spectral line|emission lines]] that span the visual spectrum,<ref>{{cite web |
|||
==Pharmacology== |
|||
|last=Talbot|first=John|url=http://web.physik.rwth-aachen.de/~harm/aixphysik/atom/discharge/index1.html |
|||
Haloperidol is a typical butyrophenone type antipsychotic that exhibits high affinity dopamine D2 receptor antagonism and slow receptor dissociation kinetics.<ref>{{cite journal|title=Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors.|journal=Mol Psychiatry. 1998 Mar;3(2):123-34.|pmid=9577836|url=http://www.ncbi.nlm.nih.gov/pubmed/9577836}}</ref> Haloperidol's neglible affinity for histamine H1 receptors and muscarinic M1 acetylcholine receptors yeilds an antipsychotic with a lower incidence of sedation, weight gain and orthostatic hypotension though higher rates of treatment emergent extra pyramidal symptoms. |
|||
|title=Spectra of Gas Discharges |
|||
|publisher=Rheinisch-Westfälische Technische Hochschule Aachen|accessdate=2006-08-10}}</ref> |
|||
but the most intense lines occur in the region of blue light, which produces the coloration.<ref>{{cite book |
|||
|first=William Marshall|last=Watts|year=1904 |
|||
|title=An Introduction to the Study of Spectrum Analysis |
|||
|publisher=Longmans, Green, and co. |
|||
|location=London}}</ref> |
|||
==Occurrence and production== |
|||
Haloperidol is an antagonist at the following receptors: (Ki) |
|||
Xenon is a [[trace gas]] in [[Earth's atmosphere]], occurring at 87±1 [[Parts-per notation|parts per billion]] (nL/L), or approximately 1 part per 11.5 million,<ref name="kirk">{{cite book |
|||
|last=Hwang|first=Shuen-Cheng |
|||
|coauthors=Robert D. Lein, Daniel A. Morgan |
|||
|chapter=Noble Gases |
|||
|title=Kirk-Othmer Encyclopedia of Chemical Technology |
|||
|publisher=Wiley|year=2005|edition=5th |
|||
|doi=10.1002/0471238961.0701190508230114.a01 |
|||
|isbn=0-471-48511-X}}</ref> and is also found in gases emitted from some [[mineral spring]]s. |
|||
Xenon is obtained commercially as a byproduct of the [[air separation|separation of air]] into [[oxygen]] and [[nitrogen]]. After this separation, generally performed by [[fractional distillation]] in a double-column plant, the [[liquid oxygen]] produced will contain small quantities of krypton and xenon. By additional fractional distillation steps, the liquid oxygen may be enriched to contain 0.1–0.2% of a krypton/xenon mixture, which is extracted either via adsorption onto [[silica gel]] or by distillation. Finally, the krypton/xenon mixture may be separated into [[krypton]] and xenon via distillation.<ref>{{cite book |
|||
Dopamine D2: 1.55nM<ref>{{cite journal|title=In vitro and in vivo receptor binding and effects on monoamine turnover in rat brain regions of the novel antipsychotics risperidone and ocaperidone.|journal=Mol Pharmacol. 1992 Mar;41(3):494-508.|pmid=1372084|url=http://www.ncbi.nlm.nih.gov/pubmed/1372084}}</ref> |
|||
|first=Frank G.|last=Kerry|year=2007 |
|||
|title=Industrial Gas Handbook: Gas Separation and Purification|pages=101–103|publisher=CRC Press |
|||
|isbn=0-8493-9005-2|url=http://books.google.com/?id=cXNmyTTGbRIC&pg=PA101 |
|||
}}</ref><ref>{{cite web |
|||
|url=http://www.c-f-c.com/specgas_products/xenon.htm |
|||
|title=Xenon – Xe|accessdate=2007-09-07 |
|||
|date=August 10, 1998|publisher=CFC StarTec LLC |
|||
}}</ref> Extraction of a liter of xenon from the atmosphere requires 220 [[watt-hour]]s |
|||
of energy.<ref name="singh">{{cite web |
|||
|last=Singh|first=Sanjay|date=May 15, 2005 |
|||
|url=http://www.expresshealthcaremgmt.com/20050515/criticare10.shtml |
|||
|title=Xenon: A modern anaesthetic |
|||
|publisher=Indian Express Newspapers Limited |
|||
|accessdate=2007-10-10 |
|||
|archiveurl=http://web.archive.org/web/20070813212536/http://www.expresshealthcaremgmt.com/20050515/criticare10.shtml <!--Added by H3llBot--> |
|||
|archivedate=2007-08-13}}</ref> Worldwide production of xenon in 1998 was estimated at 5,000–7,000 m<sup>3</sup>.<ref name="ullmann">{{cite book |
|||
|last=Häussinger|first=Peter |
|||
|coauthors=Glatthaar, Reinhard; Rhode, Wilhelm; Kick, Helmut; Benkmann, Christian; Weber, Josef; Wunschel, Hans-Jörg; Stenke, Viktor; Leicht, Edith; Stenger, Hermann |
|||
|chapter=Noble Gases |
|||
|title=Ullmann's Encyclopedia of Industrial Chemistry |
|||
|publisher=Wiley|year=2001|edition=6th |
|||
|doi=10.1002/14356007.a17_485|isbn=3-527-20165-3}}</ref> Because of its low abundance, xenon is much more expensive than the lighter noble gases—approximate prices for the purchase of small quantities in Europe in 1999 were 10 [[Euro|€]]/L for xenon, 1 €/L for krypton, and 0.20 €/L for neon.<ref name="ullmann" /> |
|||
Within the Solar System, the [[nucleon]] fraction of xenon is {{Nowrap|1.56 × 10<sup>−8</sup>}}, for an [[Abundance of the chemical elements|abundance]] of one part in 64 million of the total mass.<ref>{{cite book |
|||
Dopamine D3(Inverse agonist): 0.74nM<ref>{{cite journal|title=Agonist and inverse agonist activity at the dopamine D3 receptor measured by guanosine 5'--gamma-thio-triphosphate--35S- binding.|journal=J Pharmacol Exp Ther. 1998 Apr;285(1):119-26.|pmid=9536001|url=http://www.ncbi.nlm.nih.gov/pubmed/9536001}}</ref> |
|||
|first=David|last=Arnett|year=1996 |
|||
|title=Supernovae and Nucleosynthesis |
|||
|publisher=Princeton University Press |
|||
|location=Princeton, New Jersey |
|||
|isbn=0-691-01147-8|url=http://books.google.com/?id=PXGWGnPPo0gC&pg=PA30}}</ref> Xenon is relatively rare in the [[Sun]]'s atmosphere, on [[Earth]], and in [[asteroid]]s and [[comet]]s. The planet [[Jupiter]] has an unusually high abundance of xenon in its atmosphere; about 2.6 times as much as the Sun.<ref name="mahaffy">{{cite journal |
|||
|last=Mahaffy|first=P. R. |
|||
|coauthors=Niemann, H. B.; Alpert, A.; Atreya, S. K.; Demick, J.; Donahue, T. M.; Harpold, D. N.; Owen, T. C. |
|||
|title=Noble gas abundance and isotope ratios in the atmosphere of Jupiter from the Galileo Probe Mass Spectrometer |
|||
|journal=Journal of Geophysical Research |
|||
|year=2000|volume=105|issue=E6|pages=15061–15072 |
|||
|bibcode=2000JGR...10515061M |
|||
|doi = 10.1029/1999JE001224}}</ref> This high abundance remains unexplained and may have been caused by an early and rapid buildup of [[planetesimal]]s—small, subplanetary bodies—before the [[solar nebula|presolar disk]] began to heat up.<ref>{{cite journal |
|||
|last=Owen|first=Tobias|coauthors=Mahaffy, Paul; Niemann, H. B.; Atreya, Sushil; Donahue, Thomas; Bar-Nun, Akiva; de Pater, Imke|title=A low-temperature origin for the planetesimals that formed Jupiter |
|||
|journal=Nature|year=1999|volume=402 |
|||
|issue=6759|pages=269–70 |
|||
|bibcode=1999Natur.402..269O|doi = 10.1038/46232 |
|||
|pmid=10580497}}</ref> (Otherwise, xenon would not have been trapped in the planetesimal ices.) The problem of the low terrestrial xenon may potentially be explained by [[covalent bond]]ing of xenon to oxygen within [[quartz]], hence reducing the outgassing of xenon into the atmosphere.<ref>{{cite journal |
|||
|author=Sanloup, Chrystèle ''et al.'' |
|||
|title=Retention of Xenon in Quartz and Earth's Missing Xenon|journal=Science|year=2005|volume=310 |
|||
|issue=5751|pages=1174–7|doi=10.1126/science.1119070 |
|||
|pmid=16293758 |
|||
|bibcode = 2005Sci...310.1174S }}</ref> |
|||
Unlike the lower mass noble gases, the normal [[stellar nucleosynthesis]] process inside a star does not form xenon. Elements more massive than [[iron-56]] have a net energy cost to produce through fusion, so there is no energy gain for a star when creating xenon.<ref>{{cite book |
|||
Dopamine D4: 5-9nM<ref>{{cite journal|title=Risperidone: a novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity.|journal=J Clin Psychiatry. 1994 May;55 Suppl:5-12.|pmid=7520908|url=http://www.ncbi.nlm.nih.gov/pubmed/7520908}}</ref> |
|||
|first=Donald D.|last=Clayton|year=1983 |
|||
|title=Principles of Stellar Evolution and Nucleosynthesis |
|||
|publisher=University of Chicago Press |
|||
|isbn=0-226-10953-4|url=http://books.google.com/?id=imjwZdXExQIC&pg=PA604}}</ref> Instead, xenon is formed during [[supernova]] explosions,<ref name="heymann">{{cite conference |
|||
|last=Heymann|first=D.|coauthors=Dziczkaniec, M. |
|||
|title=Xenon from intermediate zones of supernovae |
|||
|booktitle=Proceedings 10th Lunar and Planetary Science Conference |
|||
|pages=1943–1959|publisher=Pergamon Press, Inc. |
|||
|date=March 19–23, 1979|location=Houston, Texas |
|||
|bibcode=1979LPSC...10.1943H}}</ref> by the slow neutron capture process ([[s-process]]) of [[red giant]] stars that have exhausted the hydrogen at their cores and entered the [[asymptotic giant branch]],<ref>{{cite journal |
|||
|author=Beer, H.; Kaeppeler, F.; Reffo, G.; Venturini, G. |
|||
|title=Neutron capture cross-sections of stable xenon isotopes and their application in stellar nucleosynthesis |
|||
|journal=Astrophysics and Space Science |volume=97 |
|||
|issue=1 |month=November |year=1983 |pages=95–119 |
|||
|doi=10.1007/BF00684613 |bibcode=1983Ap&SS..97...95B}}</ref> in classical [[nova]] explosions<ref>{{cite journal |
|||
|last=Pignatari|first=M. |
|||
|coauthors=Gallino, R.; Straniero, O.; Davis, A. |
|||
|title=The origin of xenon trapped in presolar mainstream SiC grains |
|||
|journal=Memorie della Societa Astronomica Italiana |
|||
|year=2004|volume=75|pages=729–734 |
|||
|bibcode=2004MmSAI..75..729P |
|||
|last2=Gallino |
|||
|last3=Straniero |
|||
|last4=Davis}}</ref> and from the radioactive decay of elements such as [[iodine]], [[uranium]] and [[plutonium]].<ref name="caldwell" /> |
|||
==Isotopes and isotopic studies== |
|||
Sigma 1(Irreversible inactivation by HPP+): 3nM<ref>{{cite journal|title=Irreversible blockade of sigma-1 receptors by haloperidol and its metabolites in guinea pig brain and SH-SY5Y human neuroblastoma cells.|journal=J Neurochem. 2007 Aug;102(3):812-25. Epub 2007 Apr 10.|pmid=17419803|url=http://www.ncbi.nlm.nih.gov/pubmed/17419803}}</ref> |
|||
{{main|Isotopes of xenon}} |
|||
Naturally occurring xenon is made of eight [[stable isotope|stable]] [[isotope]]s, the most of any element with the exception of [[tin]], which has ten. Xenon and tin are the only elements to have more than seven stable isotopes.<ref>{{cite book |
|||
Sigma 2 agonist: 54nM<ref>{{cite journal|title=Antiproliferative and cytotoxic effects of some sigma2 agonists and sigma1 antagonists in tumour cell lines.|journal=Naunyn Schmiedebergs Arch Pharmacol. 2004 Aug;370(2):106-13. Epub 2004 Jul 31.|pmid=15322732|url=http://www.ncbi.nlm.nih.gov/pubmed/15322732}}</ref> |
|||
|first=J. B.|last=Rajam|year=1960 |
|||
|title=Atomic Physics|edition=7th |
|||
|publisher=S. Chand and Co.|location=Delhi |
|||
|isbn=81-219-1809-X}}</ref> The isotopes <sup>124</sup>Xe and <sup>134</sup>Xe are predicted to undergo [[double beta decay]], but this has never been observed so they are considered to be stable.<ref>{{cite journal |
|||
|last=Barabash|first=A. S. |
|||
|title=Average (Recommended) Half-Life Values for Two-Neutrino Double-Beta Decay |
|||
|journal=Czechoslovak Journal of Physics |
|||
|year=2002|volume=52|issue=4|pages=567–573 |
|||
|doi=10.1023/A:1015369612904|arxiv = nucl-ex/0203001 |bibcode = 2002CzJPh..52..567B }}</ref> |
|||
Besides these stable forms, there are over 40 unstable isotopes that have been studied. The longest lived these isotopes is <sup>136</sup>Xe, which has been observed to undergo double beta decay with a half-life of {{nowrap|2.11 x 10<sup>21</sup>yr}}.<ref name="EXO" /> <sup>129</sup>Xe is produced by [[beta decay]] of <sup>129</sup>[[iodine|I]], which has a [[half-life]] of 16 million years, while <sup>131m</sup>Xe, <sup>133</sup>Xe, <sup>133m</sup>Xe, and <sup>135</sup>Xe are some of the [[nuclear fission|fission]] products of both <sup>235</sup>[[uranium|U]] and <sup>239</sup>[[plutonium|Pu]],<ref name="caldwell">{{cite web |
|||
|last=Caldwell|first=Eric|month=January|year=2004 |
|||
|url=http://wwwrcamnl.wr.usgs.gov/isoig/period/xe_iig.html |
|||
|title=Periodic Table – Xenon|work=Resources on Isotopes |
|||
|publisher=USGS|accessdate=2007-10-08}}</ref> and therefore used as indicators of nuclear explosions. |
|||
Nuclei of two of the stable [[isotopes of xenon]], <sup>129</sup>Xe and <sup>131</sup>Xe, have non-zero intrinsic [[angular momentum|angular momenta]] ([[Spin (physics)|nuclear spins]], suitable for [[nuclear magnetic resonance]]). The nuclear spins can be aligned beyond ordinary polarization levels by means of circularly polarized light and [[rubidium]] vapor.<ref>{{cite journal |
|||
5HT1A Agonist: 1927nM<ref name="affindata">{{cite journal|title=H1-Histamine Receptor Affinity Predicts Short-Term Weight Gain for Typical and Atypical Antipsychotic Drugs|journal=Neuropsychopharmacology (2003) 28, 519–526. doi:10.1038/sj.npp.1300027|url=http://www.nature.com/npp/journal/v28/n3/full/1300027a.html}}</ref> |
|||
|last=Otten|first=Ernst W.|year=2004 |
|||
|title=Take a breath of polarized noble gas |
|||
|journal=Europhysics News|volume=35|issue=1|doi=10.1051/epn:2004109 |
|||
|page=16|bibcode = 2004ENews..35...16O }}</ref> The resulting [[spin polarization]] of xenon [[atomic nucleus|nuclei]] can surpass 50% of its maximum possible value, greatly exceeding the equilibrium value dictated by the [[Boltzmann distribution]] (typically 0.001% of the maximum value at [[room temperature]], even in the strongest [[magnet]]s). Such non-equilibrium alignment of spins is a temporary condition, and is called ''[[hyperpolarization (physics)|hyperpolarization]]''. The process of hyperpolarizing the xenon is called ''optical pumping'' (although the process is different from [[optical pumping|pumping a laser]]).<ref>{{cite journal |
|||
|journal=Physical Review Letters|volume=96 |
|||
|issue=5|page=053002 |
|||
|year=2006|title = Optical Pumping System Design for Large Production of Hyperpolarized <sup>129</sup>Xe |
|||
|first=I. C.|last=Ruset|coauthors=Ketel, S.; Hersman, F. W.|doi=10.1103/PhysRevLett.96.053002 |bibcode=2006PhRvL..96e3002R}}</ref> |
|||
Because a <sup>129</sup>Xe nucleus has a [[spin (physics)|spin]] of 1/2, and therefore a zero [[electric field|electric]] [[quadrupole moment]], the <sup>129</sup>Xe nucleus does not experience any quadrupolar interactions during collisions with other atoms, and thus its hyperpolarization can be maintained for long periods of time even after the laser beam has been turned off and the alkali vapor removed by condensation on a room-temperature surface. Spin polarization of <sup>129</sup>Xe can persist from several [[second]]s for xenon atoms dissolved in [[blood]]<ref>{{ |
|||
5HT2A: 53nM<ref name="affindata" /> |
|||
cite journal |
|||
|first=J.|last=Wolber |
|||
|coauthors=Cherubini, A.; Leach, M. O.; Bifone, A. |
|||
|title = On the oxygenation-dependent <sup>129</sup>Xe ''T''<sub>1</sub> in blood |
|||
|year = 2000|journal = NMR in Biomedicine |
|||
|volume = 13|issue = 4|pages = 234–7 |
|||
|doi = 10.1002/1099-1492(200006)13:4<234::AID-NBM632>3.0.CO;2-K |
|||
|pmid=10867702}}</ref> to several hours in the [[gas phase]]<ref>{{ cite journal |
|||
|first=B.|last=Chann|coauthors=Nelson, I. A.; Anderson, L. W.; Driehuys, B.; Walker, T. G. |
|||
|title=<sup>129</sup>Xe-Xe molecular spin relaxation |
|||
|year=2002|journal=Physical Review Letters |
|||
|volume=88|issue=11|pages=113–201 |
|||
|doi=10.1103/PhysRevLett.88.113201 |bibcode=2002PhRvL..88k3201C}}</ref> and several days in deeply frozen solid xenon.<ref>{{cite encyclopedia |
|||
|first=Gustav Konrad|last=von Schulthess |
|||
|coauthors=Smith, Hans-Jørgen; Pettersson, Holger; Allison, David John |
|||
|year=1998|title=The Encyclopaedia of Medical Imaging |
|||
|page=194|publisher=Taylor & Francis |
|||
|isbn=1-901865-13-4|url=http://books.google.com/books?id=zvDY5unRC4oC&pg=PA194}}</ref> In contrast, [[isotopes of xenon|<sup>131</sup>Xe]] has a nuclear spin value of 3/2 and a nonzero [[quadrupole moment]], and has ''T''<sub>1</sub> relaxation times in the [[millisecond]] and [[second]] ranges.<ref>{{cite journal |
|||
|first=W. W.|last=Warren|coauthors=Norberg, R. E. |
|||
|title=Nuclear Quadrupole Relaxation and Chemical Shift of Xe<sup>131</sup> in Liquid and Solid Xenon |
|||
|year=1966|journal=Physical Review |
|||
|volume=148|issue=1|pages=402–412 |
|||
|doi=10.1103/PhysRev.148.402|bibcode = 1966PhRv..148..402W }}</ref> |
|||
Some radioactive isotopes of xenon, for example, <sup>133</sup>Xe and <sup>135</sup>Xe, are produced by [[neutron]] irradiation of fissionable material within [[nuclear reactor]]s.<ref name="lanl" /> [[Xenon-135|<sup>135</sup>Xe]] is of considerable significance in the operation of [[nuclear reactor|nuclear fission reactors]]. <sup>135</sup>Xe has a huge [[Neutron cross-section|cross section]] for [[thermal neutron]]s, 2.6×10<sup>6</sup> [[Barn (unit)|barns]],<ref name="stacey">{{cite book |
|||
5HT2C (Unknown): 10,000nM<ref name="affindata" /> |
|||
|first=Weston M.|last=Stacey|year=2007 |
|||
|title=Nuclear Reactor Physics|page=213 |
|||
|url=http://books.google.com/?id=y1UgcgVSXSkC&pg=PA213|publisher=Wiley-VCH|isbn=3-527-40679-4}}</ref> so it acts as a [[neutron absorber]] or "[[nuclear poison|poison]]" that can slow or stop the chain reaction after a period of operation. This was discovered in the earliest nuclear reactors built by the American [[Manhattan Project]] for [[plutonium]] production. Fortunately the designers had made provisions in the design to increase the reactor's reactivity (the number of neutrons per fission that go on to fission other atoms of [[nuclear fuel]]).<ref>{{cite web |
|||
|author=Staff|url=http://www.cfo.doe.gov/me70/manhattan/hanford_operational.htm |
|||
|archiveurl=http://web.archive.org/web/20091210094859/http://www.cfo.doe.gov/me70/manhattan/hanford_operational.htm |
|||
|archivedate=2009-12-10 |
|||
|title=Hanford Becomes Operational |
|||
|work=The Manhattan Project: An Interactive History |
|||
|publisher=U.S. Department of Energy |
|||
|accessdate=2007-10-10}}</ref> |
|||
<sup>135</sup>Xe reactor poisoning played a major role in the [[Chernobyl disaster#Conditions prior to the accident|Chernobyl disaster]].<ref>{{cite book |
|||
|title=Modern Physics: An Introductory Text|year=2000 |
|||
|first=Jeremy I.|last=Pfeffer|coauthors=Nir, Shlomo |
|||
|pages=421 ff.|publisher=Imperial College Press |
|||
|isbn=1-86094-250-4|url=http://books.google.com/?id=KmMYWP56t98C&pg=PA421}}</ref> A shutdown or decrease of power of a reactor can result in buildup of <sub>135</sub>Xe and getting the reactor into the [[iodine pit]]. |
|||
Under adverse conditions, relatively high concentrations of radioactive xenon isotopes may be found emanating from nuclear reactors due to the release of fission products from cracked [[fuel rod]]s,<ref>{{cite book |
|||
5HT6: 3666nM<ref name="affindata" /> |
|||
|first=Edwards A.|last=Laws|year=2000 |
|||
|title=Aquatic Pollution: An Introductory Text |
|||
|page=505|publisher=John Wiley and Sons |
|||
|isbn=0-471-34875-9|url=http://books.google.com/?id=11LI7XyEIsAC&pg=PA505}}</ref> or fissioning of uranium in [[Water cooling|cooling water]].<ref>{{cite news |
|||
|author=Staff|date=April 9, 1979 |
|||
|title=A Nuclear Nightmare|publisher=Time |
|||
|url=http://www.time.com/time/magazine/article/0,9171,920196-4,00.html |
|||
|accessdate=2007-10-09}}</ref> |
|||
Because xenon is a tracer for two parent isotopes, xenon isotope ratios in [[meteorite]]s are a powerful tool for studying the [[formation of the solar system]]. The [[Iodine-xenon dating|iodine-xenon method]] of [[Radiometric dating|dating]] gives the time elapsed between [[nucleosynthesis]] and the condensation of a solid object from the [[solar nebula]]. In 1960, physicist [[John Reynolds (physicist)|John H. Reynolds]] discovered that certain [[meteorite]]s contained an isotopic anomaly in the form of an overabundance of xenon-129. He inferred that this was a [[decay product]] of radioactive [[iodine-129]]. This isotope is produced slowly by [[cosmic ray spallation]] and [[nuclear fission]], but is produced in quantity only in supernova explosions. As the half-life of <sup>129</sup>I is comparatively short on a cosmological time scale, only 16 million years, this demonstrated that only a short time had passed between the supernova and the time the meteorites had solidified and trapped the <sup>129</sup>I. These two events (supernova and solidification of gas cloud) were inferred to have happened during the early history of the [[Solar System]], as the <sup>129</sup>I isotope was likely generated before the Solar System was formed, but not long before, and seeded the solar gas cloud with isotopes from a second source. This supernova source may also have caused collapse of the solar gas cloud.<ref>{{cite book |
|||
5HT7: 377.2nM<ref name="affindata" /> |
|||
|first=Donald D.|last=Clayton|year=1983 |
|||
|title=Principles of Stellar Evolution and Nucleosynthesis |
|||
|page=75|edition=2nd|url=http://books.google.com/?id=imjwZdXExQIC&pg=PA604 |
|||
|publisher=University of Chicago Press|isbn=0-226-10953-4}}</ref><ref>{{cite web |
|||
|author=Bolt, B. A.; Packard, R. E.; Price, P. B. |
|||
|year=2007|url=http://content.cdlib.org/xtf/view?docId=hb1r29n709&doc.view=content&chunk.id=div00061&toc.depth=1&brand=oac&anchor.id=0 |
|||
|title=John H. Reynolds, Physics: Berkeley |
|||
|publisher=The University of California, Berkeley |
|||
|accessdate=2007-10-01}}</ref> |
|||
In a similar way, xenon isotopic ratios such as <sup>129</sup>Xe/<sup>130</sup>Xe and <sup>136</sup>Xe/<sup>130</sup>Xe are also a powerful tool for understanding planetary differentiation and early outgassing.<ref name="kaneoka">{{cite journal |
|||
Histamine H1: 1800nM<ref name="affindata" /> |
|||
|last=Kaneoka|first=Ichiro|title=Xenon's Inside Story |
|||
|journal=Science|year=1998|volume=280|issue=5365 |
|||
|pages=851–852|doi=10.1126/science.280.5365.851b}}</ref> For example, The [[atmosphere of Mars]] shows a xenon abundance similar to that of Earth: |
|||
0.08 parts per million,<ref>{{cite web |
|||
|last=Williams|first=David R. |
|||
|date=September 1, 2004|url=http://nssdc.gsfc.nasa.gov/planetary/factsheet/marsfact.html |
|||
|title=Mars Fact Sheet|publisher=NASA |
|||
|accessdate=2007-10-10}}</ref> however Mars shows a higher proportion of <sup>129</sup>Xe than the Earth or the Sun. As this isotope is generated by radioactive decay, the result may indicate that Mars lost most of its primordial atmosphere, possibly within the first 100 million years after the planet was formed.<ref>{{cite web |
|||
|last=Schilling|first=James |
|||
|url=http://humbabe.arc.nasa.gov/mgcm/HTML/FAQS/thin_atm.html|title=Why is the Martian atmosphere so thin and mainly carbon dioxide? |
|||
|publisher=Mars Global Circulation Model Group |
|||
|accessdate=2007-10-10}} |
|||
</ref><ref>{{cite journal |
|||
|last=Zahnle|first=Kevin J. |
|||
|title=Xenological constraints on the impact erosion of the early Martian atmosphere |
|||
|journal=Journal of Geophysical Research |
|||
|year=1993|volume=98|issue=E6|pages=10,899–10,913 |
|||
|doi=10.1029/92JE02941 |bibcode=1993JGR....9810899Z}}</ref> In another example, excess <sup>129</sup>Xe found in [[carbon dioxide]] well gases from [[New Mexico]] was believed to be from the decay of [[Mantle (geology)|mantle]]-derived gases soon after Earth's formation.<ref name="caldwell" /><ref>{{cite journal |
|||
|last=Boulos|first=M. S.|coauthors=Manuel, O.K. |
|||
|title=The xenon record of extinct radioactivities in the Earth|journal=[[Science (journal)|Science]] |
|||
|volume=174|issue=4016|pages=1334–6|year=1971 |
|||
|doi=10.1126/science.174.4016.1334|pmid=17801897|bibcode = 1971Sci...174.1334B }}</ref> |
|||
==Compounds== |
|||
Muscarinic M1: 10,000nM<ref name="affindata" /> |
|||
{{Category see also|Xenon compounds}} |
|||
After Neil Bartlett's discovery in 1962 that xenon can form chemical compounds, a large number of xenon compounds have been discovered and described. Almost all known xenon compounds contain the [[electronegative]] atoms fluorine or oxygen.<ref name="harding1"/> |
|||
Alpha Adrenergic 1a: 12nM<ref name="affindata" /> |
|||
===Halides=== |
|||
Alpha Adrenergic 2a: 1130nM<ref name="affindata" /> |
|||
[[Image:Xenon-tetrafluoride-3D-vdW.png|thumb|[[Xenon tetrafluoride]]|alt=A model of planar chemical molecule with a blue center atom (Xe) symmetrically bonded to four peripheral atoms (fluorine).]] |
|||
[[Image:Xenon tetrafluoride.JPG|thumb|XeF<sub>4</sub> crystals, 1962|alt=Many cubic transparent crystalls in a petri dish.]] |
|||
Three [[fluoride]]s are known: [[xenon difluoride|{{chem|XeF|2}}]], [[xenon tetrafluoride|{{chem|XeF|4}}]], and [[xenon hexafluoride|{{chem|XeF|6}}]]. XeF is theorized to be unstable.<ref>{{Cite journal | title = Probable nonexistence of xenon monofluoride as a chemically bound species in the gas phase | author = Dean H Liskow, Henry F I I I Schaefer, Paul S Bagus, Bowen Liu | journal = J Amer Chem Soc | year = 1973 | volume = 95 | issue = 12 | pages = 4056-4057}}</ref> The fluorides are the starting point for the synthesis of almost all xenon compounds. |
|||
Alpha Adrenergic2b: 480nM<ref name="affindata" /> |
|||
The solid, crystalline difluoride {{chem|XeF|2}} is formed when a mixture of [[fluorine]] and xenon gases is exposed to ultraviolet light.<ref>{{cite journal |
|||
Alpha Adrenergic2c: 550nM<ref name="affindata" /> |
|||
|author=Weeks, James L.; Chernick, Cedric; Matheson, Max S. |
|||
|title=Photochemical Preparation of Xenon Difluoride |
|||
|journal=Journal of the American Chemical Society |
|||
|volume=84 |
|||
|issue=23|page=4612|doi=10.1021/ja00882a063 |
|||
|year=1962}}</ref> Ordinary daylight is sufficient.<ref>{{cite journal |
|||
|author=Streng, L. V.; Streng, A. G. |
|||
|title=Formation of Xenon Difluoride from Xenon and Oxygen Difluoride or Fluorine in Pyrex Glass at Room Temperature|journal=Inorganic Chemistry |
|||
|year=1965|volume=4|issue=9|pages=1370–1371 |
|||
|doi=10.1021/ic50031a035}}</ref> Long-term heating of {{chem|XeF|2}} at high temperatures under an {{chem|NiF|2}} catalyst yields {{chem|XeF|6}}.<ref name="tramsek">{{cite journal |
|||
|author=Tramšek, Melita; Žemva, Boris |
|||
|title=Synthesis, Properties and Chemistry of Xenon(II) Fluoride|journal=Acta Chimica Slovenica |
|||
|date=December 5, 2006|volume=53|issue=2 |
|||
|pages=105–116|format=PDF|doi=10.1002/chin.200721209 |
|||
|url=http://acta.chem-soc.si/53/53-2-105.pdf |
|||
|accessdate=2009-07-18}}</ref> Pyrolysis of {{chem|XeF|6}} in the presence of [[sodium fluoride|NaF]] yields high-purity {{chem|XeF|4}}.<ref>{{cite journal |
|||
|author=Ogrin, Tomaz; Bohinc, Matej; Silvnik, Joze |
|||
|title=Melting-point determinations of xenon difluoride-xenon tetrafluoride mixtures |
|||
|journal=Journal of Chemical and Engineering Data |
|||
|year=1973|volume=18|issue=4|page=402 |
|||
|doi=10.1021/je60059a014}}</ref> |
|||
The xenon fluorides behave as both fluoride acceptors and fluoride donors, forming salts that contain such cations as {{chem|XeF|+}} and Xe<sub>2</sub>F<sub>3</sub><sup>+</sup>, and anions such as XeF<sub>5</sub><sup>−</sup>, XeF<sub>7</sub><sup>−</sup>, and XeF<sub>8</sub><sup>2−</sup>. The green, paramagnetic Xe<sub>2</sub><sup>+</sup> is formed by the reduction of {{chem|XeF|2}} by xenon gas.<ref name="harding1">{{cite book|author=Harding, Charlie; Johnson, David Arthur; Janes, Rob|title = Elements of the ''p'' block |
|||
NR1\NR2B Subunit containing NMDA receptor antagonist (Ifenprodil site): IC50 2mM<ref>{{cite journal|title=Subtype-selective inhibition of N-methyl-D-aspartate receptors by haloperidol.|journal=Mol Pharmacol. 1996 Dec;50(6):1541-50.|pmid=8967976|url=http://www.ncbi.nlm.nih.gov/pubmed/8967976}}</ref> |
|||
|pages=93–94|publisher=Royal Society of Chemistry |
|||
|location=Great Britain|year=2002 |
|||
|isbn=0-85404-690-9|url=http://books.google.com/?id=W0HW8wgmQQsC&pg=PA93}}</ref> |
|||
{{chem|XeF|2}} is also able to form [[complex (chemistry)|coordination complex]]es with transition metal ions. Over 30 such complexes have been synthesized and characterized.<ref name="tramsek" /> |
|||
==Pharmacokinetics== |
|||
Haloperidol has a half-life of 17 hours when administered orally and 15 hours when administered IM or IV. Haloperidol has an average oral bioavailability of 65%.<ref>{{cite journal|title=Elimination half-life and bioavailability of haloperidol in schizophrenic patients.|journal=J Clin Psychiatry. 1985 Jan;46(1):20-1.|pmid=3965439|url=http://www.ncbi.nlm.nih.gov/pubmed/3965439}}</ref> |
|||
Haloperidol and reduced haloperidol are eliminated extensively via gluconuride conjugation.<ref>{{cite journal|title=Haloperidol metabolism in psychiatric patients: importance of glucuronidation and carbonyl reduction.|journal=J Clin Psychopharmacol. 1992 Jun;12(3):169-74.|pmid=1629382|url=http://www.ncbi.nlm.nih.gov/pubmed/1629382}}</ref> |
|||
Haloperidol undergoes extensive hepatic microsomal biotransformation primarily via CYP450 3A4 & 2D6 and carbonyl reductase. CYP450 3A4 activity governs metabolism to neurotoxic pyridinium metabolites.<ref>{{cite journal|title=Assessment of the contributions of CYP3A4 and CYP3A5 in the metabolism of the antipsychotic agent haloperidol to its potentially neurotoxic pyridinium metabolite and effect of antidepressants on the bioactivation pathway.|journal=Drug Metab Dispos. 2003 Mar;31(3):243-9.|pmid=12584149|url=http://www.ncbi.nlm.nih.gov/pubmed/12584149}}</ref> |
|||
Whereas the xenon fluorides are well-characterized, the other halides are not known, the only exception being the dichloride, [[Xenon dichloride|XeCl<sub>2</sub>]]. Xenon dichloride is reported to be an endothermic, colorless, crystalline compound that decomposes into the elements at 80°C, formed by the high-frequency irradiation of a mixture of xenon, fluorine, and [[silicon tetrachloride|silicon]] or [[carbon tetrachloride]].<ref name="scott1">{{cite encyclopedia |
|||
[File:Haloperidol (Haldol).jpg|thumb|Haloperidol for injection]] |
|||
|author=Scott, Thomas; Eagleson, Mary |
|||
|title = Xenon Compounds|year=1994 |
|||
|encyclopedia=Concise encyclopedia chemistry |
|||
|publisher=Walter de Gruyter |
|||
|isbn=3-11-011451-8|url=http://books.google.com/books?id=Owuv-c9L_IMC&pg=PA1183|page=1183}}</ref> However, doubt has been raised as to whether {{chem|XeCl|2}} is a real compound and not merely a [[van der Waals molecule]] consisting of weakly bound Xe atoms and {{chem|Cl|2}} molecules.<ref>{{cite journal |
|||
|author=Proserpio, Davide M.; Hoffmann, Roald; Janda, Kenneth C. |
|||
|title=The xenon-chlorine conundrum: van der Waals complex or linear molecule?|year=1991|volume=113 |
|||
|journal=Journal of the American Chemical Society |
|||
|issue=19 |
|||
|page=7184|doi=10.1021/ja00019a014}}</ref> Theoretical calculations indicate that the linear molecule {{chem|XeCl|2}} is less stable than the van der Waals complex.<ref>{{cite journal |
|||
|author=Richardson, Nancy A.; Hall, Michael B.|year=1993 |
|||
|title=The potential energy surface of xenon dichloride |
|||
|journal=The Journal of Physical Chemistry|volume=97 |
|||
|issue=42 |
|||
|page=10952|doi=10.1021/j100144a009}}</ref> |
|||
=== |
===Oxides and oxohalides=== |
||
Three oxides of xenon are known: [[xenon trioxide]] ({{chem|XeO|3}}) and [[xenon tetroxide]] ({{chem|XeO|4}}), both of which are dangerously explosive and powerful oxidizing agents, and [[xenon dioxide]] (XeO<sub>2</sub>), which was reported in 2011 with a [[coordination number]] of four.<ref>{{cite journal |
|||
Plasma levels of four to 25 micrograms per liter are required for therapeutic action. The determination of plasma levels can be used to calculate dose adjustments and to check compliance, particularly in long-term patients. Plasma levels in excess of the therapeutic range may lead to a higher incidence of side effects or even pose the risk of haloperidol intoxication. |
|||
|author=Brock, D.S..; Schrobilgen, G.J. |
|||
|title=Synthesis of the missing oxide of xenon, XeO<sub>2</sub>, and its implications for earth's missing xenon |
|||
|journal=[[Journal of the American Chemical Society]] |
|||
|page=110222081739042 |
|||
|year=2011 |doi=10.1021/ja110618g |
|||
|volume=133 |
|||
|issue=16 |
|||
|pmid=21341650}}</ref> XeO<sub>2</sub> forms when xenon tetrafluoride is poured over ice. Its crystal structure may allow it to replace silicon in silicate minerals.<ref name="ChemistryWhere2011">{{cite doi|10.1038/471138d}}</ref> The XeOO<sup>+</sup> cation has been identified by [[infrared spectroscopy]] in solid [[argon]].<ref>{{cite journal |
|||
|author=Zhou, M.; Zhao, Y.; Gong, Y.; Li, J. |
|||
|title=Formation and Characterization of the XeOO<sup>+</sup> Cation in Solid Argon |
|||
|journal=[[Journal of the American Chemical Society]] |
|||
|year=2006|volume=128 |
|||
|issue=8 |
|||
|pmid=16492012|pages=2504–5 |
|||
|doi= 10.1021/ja055650n}}</ref> |
|||
Xenon does not react with oxygen directly; the trioxide is formed by the hydrolysis of {{chem|XeF|6}}:<ref>{{cite book |
|||
The concentration of haloperidol in brain tissue is about 20-fold higher compared to blood levels. It is slowly eliminated from brain tissue,<ref name="Kornhuber1999">Kornhuber J, Schultz A, Wiltfang J, Meineke I, Gleiter CH, Zöchling R, Boissl KW, Leblhuber F, Riederer P. Persistence of haloperidol in human brain tissue. Am.J.Psychiatry 156:885-890, 1999. PMID 10360127</ref> which may explain the slow disappearance of side effects when the medication is stopped.<ref name="Kornhuber1999" /><ref>Kornhuber J, Wiltfang J, Riederer P, Bleich S. Neuroleptic drugs in the human brain: clinical impact of persistence and region-specific distribution. Eur.Arch.Psychiatry Clin.Neurosci. 256:274-280, 2006. PMID 16788768</ref> |
|||
|first=John H.|last=Holloway|coauthors=Hope, Eric G. |
|||
|editor=A. G. Sykes|year=1998|publisher=Academic |
|||
|title=Advances in Inorganic Chemistry Press |
|||
|isbn=0-12-023646-X|page=65|url=http://books.google.com/?id=6iqXRtz6p3QC&pg=PA65}}</ref> |
|||
:{{chem|XeF|6}} + 3 {{chem|H|2|O}} → {{chem|XeO|3}} + 6 HF |
|||
==Uses== |
|||
A comprehensive review of haloperidol has found it to be an effective agent in treatment of symptoms associated with [[schizophrenia]].<ref name="pmid17054159">{{cite journal |author=Joy CB, Adams CE, Lawrie SM |title=Haloperidol versus placebo for schizophrenia |journal=Cochrane Database Syst Rev |volume= |issue=4 |pages=CD003082 |year=2006 |pmid=17054159 |doi=10.1002/14651858.CD003082.pub2|url=http://mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD003082/frame.html |editor1-last=Irving |editor1-first=Claire B}}</ref> It is also used in the control of the symptoms of: |
|||
{{chem|XeO|3}} is weakly acidic, dissolving in alkali to form unstable ''xenate'' salts containing the {{chem|HXeO|4|−}} anion. These unstable salts easily [[disproportionation|disproportionate]] into xenon gas and ''[[perxenate]]'' salts, containing the {{chem|XeO|6|4−}} anion.<ref name="henderson">{{cite book |
|||
* Acute [[psychosis]], such as drug-induced psychosis (LSD, psilocybin, amphetamines, ketamine,<ref>{{cite journal |
|||
|first=W.|last=Henderson |
|||
| author = Giannini AJ, Underwood NA, Condon M |
|||
|title = Main group chemistry|year=2000 |
|||
| title = Acute ketamine intoxication treated by haloperidol: a preliminary study |
|||
|publisher=Royal Society of Chemistry|pages=152–153 |
|||
| journal = American Journal of Therapeutics |
|||
|location=Great Britain|isbn=0-85404-617-8 |
|||
| volume = 7 |
|||
|url=http://books.google.com/?id=twdXz1jfVOsC&pg=PA152}}</ref> |
|||
| issue = 6 |
|||
| pages = 389–92 |
|||
| year = 2000 |
|||
| month = November |
|||
| pmid = 11304647 |
|||
| doi = 10.1097/00045391-200007060-00008 |
|||
}}</ref> and phencyclidine,<ref>{{cite journal |
|||
| author = Giannini AJ, Eighan MS, Loiselle RH, Giannini MC |
|||
| title = Comparison of haloperidol and chlorpromazine in the treatment of phencyclidine psychosis |
|||
| journal = Journal of Clinical Pharmacology |
|||
| volume = 24 |
|||
| issue = 4 |
|||
| pages = 202–4 |
|||
| year = 1984 |
|||
| month = April |
|||
| pmid = 6725621 |
|||
| doi = |
|||
}}</ref> and psychosis associated with high fever or metabolic disease |
|||
* Acute [[mania|manic phases]] until the concomitantly given first-line drugs such as [[Lithium pharmacology|lithium]] or [[valproate]] are effective |
|||
* [[Hyperactivity]], [[aggression]] |
|||
* Acute [[delirium]] |
|||
* Otherwise uncontrollable, severe behavioral disorders in children and adolescents |
|||
* Agitation and confusion associated with cerebral [[Sclerosis (medicine)|sclerosis]] |
|||
* Adjunctive treatment of alcohol and opioid withdrawal |
|||
* Treatment of severe nausea and emesis in postoperative and [[palliative care]], especially for palliating adverse effects of [[radiation therapy]] and [[chemotherapy]] in [[oncology]] |
|||
* Treatment of neurological disorders, such as [[tic disorder]]s, [[Tourette syndrome]], and [[Chorea (disease)|chorea]] |
|||
* Adjunctive treatment of severe chronic pain, always with [[analgesic]]s |
|||
* Therapeutic trial in personality disorders, such as [[borderline personality disorder]] |
|||
* Treatment of intractable [[hiccups]] |
|||
* Also used in aquaculture to block dopamine receptors to enable GnrHA function for ovulation use in spawning fish |
|||
Barium perxenate, when treated with concentrated [[sulfuric acid]], yields gaseous xenon tetroxide:<ref name="scott1" /> |
|||
Some weeks or even months of treatment may be needed before a remission of schizophrenia is evident. |
|||
: {{chem|Ba|2|XeO|6}} + 2 {{chem|H|2|SO|4}} → 2 {{chem|BaSO|4}} + 2 {{chem|H|2|O}} + {{chem|XeO|4}} |
|||
In some clinics, the use of [[Atypical antipsychotic|atypical neuroleptics]] (e.g., [[clozapine]], [[risperidone]], [[olanzapine]], or [[ziprasidone]]) is, in general, preferred over haloperidol, because these drugs have an appreciably lower incidence of extrapyramidal side effects. Each of these drugs, however, has its own spectrum of potentially serious side effects (e.g., agranulocytosis with clozapine, weight gain with increased risk of diabetes and of stroke). Atypical neuroleptics are also much more expensive and have recently been the subject of increasing controversy regarding their efficacy in comparison to older products and their side effects. |
|||
To prevent decomposition, the xenon tetroxide thus formed is quickly cooled to form a pale-yellow solid. It explodes above −35.9 °C into xenon and oxygen gas. |
|||
Haloperidol was considered indispensable for treating psychiatric emergency situations,<ref name="pmid3736271">{{cite journal |author=Cavanaugh SV |title=Psychiatric emergencies |journal=Med. Clin. North Am. |volume=70 |issue=5 |pages=1185–202 |year=1986 |pmid=3736271 |doi=}}</ref><ref name="pmid15985915">{{cite journal |author=Currier GW |title=The controversy over "chemical restraint" in acute care psychiatry |journal=J Psychiatr Pract |volume=9 |issue=1 |pages=59–70 |year=2003 |pmid=15985915|doi=10.1097/00131746-200301000-00006}}</ref> although the newer atypical drugs have gained greater role in a number of situations as outlined in a series of consensus reviews published between 2001 and 2005.<ref name="pmid17054159"/><ref name="pmid11500996">{{cite journal |author=Allen MH, Currier GW, Hughes DH, Reyes-Harde M, Docherty JP |title=The Expert Consensus Guideline Series. Treatment of behavioral emergencies |journal=Postgrad Med |volume= |issue=Spec No |pages=1–88; quiz 89–90 |year=2001 |pmid=11500996 |doi=}}</ref><ref name="pmid15985913">{{cite journal |author=Allen MH, Currier GW, Hughes DH, Docherty JP, Carpenter D, Ross R |title=Treatment of behavioral emergencies: a summary of the expert consensus guidelines |journal=J Psychiatr Pract |volume=9 |issue=1 |pages=16–38 |year=2003 |pmid=15985913|doi=10.1097/00131746-200301000-00004}}</ref><ref name="pmid16319571">{{cite journal |author=Allen MH, Currier GW, Carpenter D, Ross RW, Docherty JP |title=The expert consensus guideline series. Treatment of behavioral emergencies 2005 |journal=J Psychiatr Pract |volume=11 Suppl 1 |issue= |pages=5–108; quiz 110–2 |year=2005 |pmid=16319571 |doi=10.1097/00131746-200511001-00002}}</ref> It is enrolled in the [[World Health Organization]] [[List of Essential Medicines]]. |
|||
A number of xenon oxyfluorides are known, including {{chem|XeOF|2}}, [[xenon oxytetrafluoride|{{chem|XeOF|4}}]], {{chem|XeO|2|F|2}}, and {{chem|XeO|3|F|2}}. {{chem|XeOF|2}} is formed by the reaction of [[oxygen difluoride|{{chem|OF|2}}]] with xenon gas at low temperatures. It may also be obtained by the partial hydrolysis of {{chem|XeF|4}}. It disproportionates at −20 °C into {{chem|XeF|2}} and {{chem|XeO|2|F|2}}.<ref name="mackay1">{{cite book |
|||
Like many typical antipsychotics, haloperidol is more effective in treating the "positive" symptoms of schizophrenia (delusions, hallucinations, etc.) than "negative" symptoms (affective flattening, apathy, social withdrawal). |
|||
|author=Mackay, Kenneth Malcolm; Mackay, Rosemary Ann; Henderson, W. |
|||
|title=Introduction to modern inorganic chemistry |
|||
|year=2002|edition=6th|publisher=CRC Press |
|||
|isbn=0-7487-6420-8|url=http://books.google.com/?id=LpJPWKT3PNcC&pg=PA497|pages=497–501}}</ref> {{chem|XeOF|4}} is formed by the partial hydrolysis of {{chem|XeF|6}},<ref>{{cite journal |
|||
|last=Smith|first=D. F.|year=1963 |
|||
|title=Xenon Oxyfluoride |
|||
|pmid=17810680|journal=Science|volume=140 |
|||
|doi=10.1126/science.140.3569.899 |
|||
|issue=3569|page=899 |bibcode=1963Sci...140..899S |
|||
|pages=899–900}}</ref> or the reaction of {{chem|XeF|6}} with sodium perxenate, {{chem|Na|4|XeO|6}}. The latter reaction also produces a small amount of {{chem|XeO|3|F|2}}. {{chem|XeOF|4}} reacts with [[caesium fluoride|CsF]] to form the {{chem|XeOF|5|−}} anion,<ref name="mackay1" /><ref>{{ cite journal | title = On the Structure of the [XeOF<sub>5</sub>]<sup>−</sup> Anion and of Heptacoordinated Complex Fluorides Containing One or Two Highly Repulsive Ligands or Sterically Active Free Valence Electron Pairs | author = K. O. Christe, D. A. Dixon, J. C. P. Sanders, G. J. Schrobilgen, S. S. Tsai, W. W. Wilson | journal = [[Inorganic Chemistry (journal)|Inorg. Chem.]] | year = 1995 | volume = 34 | issue = 7 | pages = 1868–1874 | doi = 10.1021/ic00111a039 }}</ref> while XeOF<sub>3</sub> reacts with the alkali metal fluorides [[potassium fluoride|KF]], [[rubidium fluoride|RbF]] and CsF to form the {{chem|XeOF|4|−}} anion.<ref>{{ cite journal | title = Chlorine trifluoride oxide. V. Complex formation with Lewis acids and bases | author = K. O. Christe, C. J. Schack, D. Pilipovich | journal = [[Inorganic Chemistry (journal)|Inorg. Chem.]] | year = 1972 | volume = 11 | issue = 9 | pages = 2205–2208 | doi = 10.1021/ic50115a044 }}</ref> |
|||
===Other compounds=== |
|||
A multiple-year UK study by the Alzheimer's Research Trust suggested this drug and other [[neuroleptic]] antipsychotic drugs commonly given to Alzheimer's patients with mild behavioural problems often make their condition worse.<ref> |
|||
{{cite journal |
|||
| author = Ballard C |
|||
| title = A randomised, blinded, placebo-controlled trial in dementia patients continuing or stopping neuroleptics (the DART-AD trial) |
|||
| journal = PLoS Medicine |
|||
| volume = 5 |
|||
| issue = 4 |
|||
| pages = e76 |
|||
| year = 2008 |
|||
| month = April |
|||
| pmid = 18384230 |
|||
| pmc = 2276521 |
|||
| doi = 10.1371/journal.pmed.0050076 |
|||
| laysummary = http://news.bbc.co.uk/1/hi/health/7319393.stm |
|||
| laysource = BBC News |
|||
| laydate = 2008-04-01 |
|||
| quote=Neuroleptics provided no benefit for patients with mild behavioural problems, but were associated with a marked deterioration in verbal skills |
|||
| author-separator = , |
|||
| author2 = Lana MM |
|||
| author3 = Theodoulou M |
|||
| display-authors = 3 |
|||
| editor1-last = Brayne |
|||
| editor1-first = Carol |
|||
| last4 = Douglas |
|||
| first4 = Simon |
|||
| last5 = McShane |
|||
| first5 = Rupert |
|||
| last6 = Jacoby |
|||
| first6 = Robin |
|||
| last7 = Kossakowski |
|||
| first7 = Katja |
|||
| last8 = Yu |
|||
| first8 = Ly-Mee |
|||
| last9 = Juszczak |
|||
| first9 = Edmund |
|||
}}</ref> The study concluded that |
|||
Recently, there has been an interest in xenon compounds where xenon is directly bonded to a less electronegative element than fluorine or oxygen, particularly [[carbon]].<ref>{{cite book |
|||
{{cquote|For most patients with AD, withdrawal of neuroleptics had no overall detrimental effect on functional and cognitive status and by some measures improved functional and cognitive status. Neuroleptics may have some value in the maintenance treatment of more severe neuropsychiatric symptoms, but this possibility must be weighed against the unwanted effects of therapy. The current study helps to inform a clinical management strategy for current practice, but the considerable risks of maintenance therapy highlight the urgency of further work to find, develop, and implement safer and more effective treatment approaches for neuropsychiatric symptoms in people with AD.}} |
|||
|title=Advances in Inorganic Chemistry |
|||
|author=Holloway, John H.; Hope, Eric G. |
|||
|others=Contributor A. G. Sykes|publisher=Academic Press |
|||
|year=1998|isbn=0-12-023646-X|url=http://books.google.com/?id=6iqXRtz6p3QC&pg=PA61|pages=61–90}}</ref> Electron-withdrawing groups, such as groups with fluorine substitution, are necessary to stabilize these compounds.<ref name="henderson" /> Numerous such compounds have been characterized, including:<ref name="mackay1" /><ref>{{cite journal |
|||
|title=C<sub>6</sub>F<sub>5</sub>XeF, a versatile starting material in xenon–carbon chemistry |
|||
|year=2004|last=Frohn|first=H |
|||
|journal=Journal of Fluorine Chemistry|volume=125 |
|||
|issue=6|page=981 |
|||
|doi=10.1016/j.jfluchem.2004.01.019}}</ref> |
|||
*{{chem|C|6|F|5|–Xe|+|–N≡C–CH|3}}, where C<sub>6</sub>F<sub>5</sub> is the pentafluorophenyl group. |
|||
=== Controversial nonmedical uses === |
|||
*{{chem|[C|6|F|5|]|2|Xe}} |
|||
{{See also|Punitive psychiatry in the Soviet Union}} |
|||
*{{chem|C|6|F|5|–Xe–X}}, where X is [[nitrile|CN]], F, or Cl. |
|||
*{{chem|R–C≡C–Xe|+}}, where R is {{chem|C|2|F|5|-}} or [[butyl|''tert''-butyl]]. |
|||
*{{chem|C|6|F|5|–XeF|2|+}} |
|||
*{{chem|(C|6|F|5|Xe)|2|Cl|+}} |
|||
Other compounds containing xenon bonded to a less electronegative element include {{chem|F–Xe–N(SO|2|F)|2}} and {{chem|F–Xe–BF|2}}. The latter is synthesized from [[dioxygenyl]] tetrafluoroborate, {{chem|O|2|BF|4}}, at −100 °C.<ref name="mackay1" /><ref>{{cite journal |
|||
[[Soviet dissident]]s, including medical staff, have reported several times on the use of haloperidol in the Soviet Union for punitive purposes or simply to break the prisoners' wills.<ref>{{cite book | last = Podrabinek | first = Aleksandr | title = Punitive Medicine | publisher = Karoma Publishers | location = Ann Arbor Mich. | year = 1980 | isbn = 0-89720-022-5 | pages= 15–20}}</ref><ref name="pmid7820004">{{cite journal |
|||
|doi=10.1021/ja00764a022|title=Reaction of xenon with dioxygenyl tetrafluoroborate. Preparation of FXe-BF<sub>2</sub> |
|||
| author = Kosserev I, Crawshaw R |
|||
|year=1972 |
|||
| title = Medicine and the Gulag |
|||
|last=Goetschel|first=Charles T.|coauthors=Loos, Karl R. |
|||
| journal = BMJ (Clinical Research Ed.) |
|||
|journal=Journal of the American Chemical Society |
|||
| volume = 309 |
|||
|volume=94 |
|||
| issue = 6970 |
|||
|issue=9|page=3018}}</ref> |
|||
| pages = 1726–30 |
|||
| year = 1994 |
|||
| pmid = 7820004 |
|||
| pmc = 2542687 |
|||
}}</ref><ref>{{cite book | last = de Boer | first = S. P. | coauthors = E. J. Driessen, H. L. Verhaar | title = Biographical Dictionary of Dissidents in the Soviet Union, 1956-1975 | publisher = Martinus Nijhoff Publishers | location = The Hague | year = 1982 | isbn = 90-247-2538-0 }}</ref> Notable dissidents who were administered haloperidol as part of their court-ordered treatment were [[Sergei Kovalev]] and [[Leonid Plyushch]].<ref>{{cite journal |
|||
| author = Wade N |
|||
| title = Sergei Kovalev: Biologist Denied Due Process and Medical Care |
|||
| journal = Science |
|||
| volume = 194 |
|||
| issue = 4265 |
|||
| pages = 585–587 |
|||
| year = 1976 |
|||
| month = November |
|||
| pmid = 17818411 |
|||
| doi = 10.1126/science.194.4265.585 |
|||
}}</ref> The accounts Plyushch gave in the West, after he was allowed to leave the Soviet Union in 1976, were instrumental in triggering Western condemnation of Soviet practices at the [[World Psychiatric Association]]'s 1977 meeting.<ref>{{cite news |
|||
|title = Censuring the Soviets |
|||
|url = http://www.time.com/time/magazine/article/0,9171,915433,00.html |
|||
|work = TIME |
|||
|publisher = CNN |
|||
|date = 1977-09-12 |
|||
|accessdate = 2009-06-21 |
|||
}} |
|||
</ref> The use of haloperidol in the Soviet Union's psychiatric system was prevalent because it was one of the few psychotropic drugs produced in quantity in the USSR.<ref>[http://www.time.com/time/printout/0,8816,922041,00.html The Children of Pavlov], TIME, Jun. 23, 1980</ref> |
|||
An unusual ion containing xenon is the [[tetraxenonogold(II)]] cation, {{chem|AuXe|4|2+}}, which contains Xe–Au bonds.<ref name="waikeeli2">{{cite book |
|||
Haloperidol has been used for its sedating effects during the deportations of immigrants by the United States [[Immigration and Customs Enforcement]] (ICE). During 2002-2008, federal immigration personnel used haloperidol to sedate 356 deportees. By 2008, following court challenges over the practice, it was given to only three detainees. Following lawsuits, U.S. officials changed the procedure so the drug is administered only by the recommendation of medical personnel and under court order.<ref>{{cite news |
|||
|title = Advanced Structural Inorganic Chemistry |
|||
| title = Fewer US deportees being sedated for removal |
|||
|author=Li, Wai-Kee; Zhou, Gong-Du; Mak, Thomas C. W. |
|||
| url = http://www.epilepsy.com/newsfeeds/view/5974 |
|||
|editors=Gong-Du Zhou; Thomas C. W. Mak |
|||
| agency = Associated Press |
|||
| |
|publisher=Oxford University Press|year=2008 |
||
|isbn=0-19-921694-0|url=http://books.google.com/?id=2qAa5hp6KX4C&pg=PA678|page=678}}</ref> This ion occurs in the compound {{chem|AuXe|4|(Sb|2|F|11|)|2}}, and is remarkable in having direct chemical bonds between two notoriously unreactive atoms, xenon and [[gold]], with xenon acting as a transition metal ligand. |
|||
| date = 2008-12-30 |
|||
| accessdate = 2009-06-21 |
|||
}} |
|||
</ref><ref name=seat>{{cite news |
|||
| title = U.S. cuts back on sedating deportees with Haldol |
|||
| first = Dianne |
|||
| last = Solis |
|||
| url = http://seattletimes.nwsource.com/html/nationworld/2008590327_deport05.html |
|||
| publisher = Seattle Times |
|||
| date = 2009-01-05 |
|||
| accessdate = 2009-06-21 |
|||
}} |
|||
</ref> |
|||
In 1995, M. Räsänen and co-workers, scientists at the [[University of Helsinki]] in [[Finland]], announced the preparation of xenon dihydride (HXeH), and later xenon hydride-hydroxide (HXeOH), hydroxenoacetylene (HXeCCH), and other Xe-containing molecules.<ref>{{cite journal |
|||
==Contraindications== |
|||
|last=Gerber|first=R. B.|year=2004 |
|||
===Absolute=== |
|||
|doi=10.1146/annurev.physchem.55.091602.094420 |
|||
* Pre-existing [[coma]], acute stroke |
|||
|title=Formation of novel rare-gas molecules in low-temperature matrices |
|||
* Severe intoxication with alcohol or other central depressant drugs |
|||
|journal=Annual Review of Physical Chemistry |
|||
* Known allergy against haloperidol or other butyrophenones or other drug ingredients |
|||
|volume=55 |
|||
* Known heart disease, when combined will tend towards cardiac arrest |
|||
|issue=1|pages=55–78|pmid=15117247|bibcode = 2004ARPC...55...55G }}</ref> In 2008, Khriachtchev ''et al.'' reported the preparation of HXeOXeH by the [[photolysis]] of water within a [[cryogenic]] xenon matrix.<ref>{{cite journal |
|||
|last=Khriachtchev|first=Leonid |
|||
|coauthors=Isokoski, Karoliina; Cohen, Arik; Räsänen, Markku; Gerber, R. Benny |
|||
|title=A Small Neutral Molecule with Two Noble-Gas Atoms: HXeOXeH |
|||
|journal=Journal of the American Chemical Society |
|||
|year=2008|volume=130|issue=19|pages=6114–8 |
|||
|doi=10.1021/ja077835v|pmid=18407641}}</ref> [[Deuterium|Deuterated]] molecules, HXeOD and DXeOH, have also been produced.<ref>{{cite journal |
|||
|last=Pettersson|first=Mika|coauthors=Khriachtchev, Leonid; Lundell, Jan; Räsänen, Markku |
|||
|title=A Chemical Compound Formed from Water and Xenon: HXeOH|year=1999 |
|||
|journal=Journal of the American Chemical Society |
|||
|volume=121|issue=50|pages=11904–11905 |
|||
|doi=10.1021/ja9932784}}</ref> |
|||
===Clathrates and excimers=== |
|||
=== Special caution needed === |
|||
In addition to compounds where xenon forms a [[chemical bond]], xenon can form [[clathrate]]s—substances where xenon atoms are trapped by the [[Crystal structure|crystalline lattice]] of another compound. An example is [[xenon hydrate]] (Xe·5.75 H<sub>2</sub>O), where xenon atoms occupy vacancies in a lattice of water molecules.<ref>{{cite journal |
|||
* Pre-existing [[Parkinson's disease]]<ref>{{cite journal |
|||
|doi=10.1126/science.134.3471.15|title=A molecular theory of general anesthesia |
|||
| author = Leentjens AF, van der Mast RC |
|||
|authorlink=Linus Pauling|journal=Science|volume=134 |
|||
| title = Delirium in elderly people: an update |
|||
|issue=3471 |
|||
| journal = Current Opinion in Psychiatry |
|||
|year=1961|pages=15–21|pmid=13733483 |
|||
| volume = 18 |
|||
|last=Pauling|first=L.|bibcode = 1961Sci...134...15P }} Reprinted as {{cite book |
|||
| issue = 3 |
|||
|pages=1328–1334|title=Linus Pauling: Selected Scientific Papers|volume=2|editor=Pauling, Linus; Kamb, Barclay |
|||
| pages = 325–30 |
|||
|place=River Edge, New Jersey|publisher=World Scientific |
|||
| year = 2005 |
|||
|year=2001|isbn=981-02-2940-2|url=http://books.google.com/?id=2QduA19d_X8C&pg=PA1329}}</ref> This clathrate has a melting point of 24 °C.<ref name="henderson2">{{cite book |
|||
| month = May |
|||
|title=Main group chemistry |
|||
| pmid = 16639157 |
|||
|last=Henderson|first=W.|year=2000 |
|||
| doi = 10.1097/01.yco.0000165603.36671.97 |
|||
|publisher=Royal Society of Chemistry |
|||
| url = http://www.medscape.com/viewarticle/503089_6 |
|||
|location=Great Britain|isbn=0-85404-617-8 |
|||
| accessdate = 2009-06-21 |
|||
|url=http://books.google.com/?id=twdXz1jfVOsC&pg=PA148|page=148}}</ref> The [[deuterate]]d version of this hydrate has also been produced.<ref>{{cite journal |
|||
}}</ref> or [[dementia with Lewy bodies]] |
|||
|first=Tomoko|last=Ikeda|coauthors=Mae, Shinji; Yamamuro, Osamu; Matsuo, Takasuke; Ikeda, Susumu; Ibberson, Richard M. |
|||
* Patients at special risk for the development of [[Long QT syndrome|QT prolongation]] ([[hypokalemia]], concomitant use of other drugs causing QT prolongation) |
|||
|title=Distortion of Host Lattice in Clathrate Hydrate as a Function of Guest Molecule and Temperature |
|||
* Compromised [[liver]] function (as haloperidol is metabolized and eliminated mainly by the liver) |
|||
|journal=Journal of Physical Chemistry A |
|||
* In patients with hyperthyreosis, the action of haloperidol is intensified and side effects are more likely. |
|||
|date=November 23, 2000|volume=104|issue=46 |
|||
* IV injections: risk of hypotension or orthostatic collapse |
|||
|pages=10623–10630|doi=10.1021/jp001313j}}</ref> Such [[clathrate hydrate]]s can occur naturally under conditions of high pressure, |
|||
such as in [[Lake Vostok]] underneath the [[Antarctica|Antarctic]] ice sheet.<ref>{{cite journal |
|||
|last=McKay|first=C. P.|coauthors=Hand, K. P.; Doran, P. T.; Andersen, D. T.; Priscu, J. C. |
|||
|title=Clathrate formation and the fate of noble and biologically useful gases in Lake Vostok, Antarctica |
|||
|journal=Geophysical Letters|year=2003 |
|||
|volume=30|issue=13|page=35|doi=10.1029/2003GL017490 |bibcode=2003GeoRL..30m..35M}}</ref> Clathrate formation can be used to fractionally distill xenon, argon and krypton.<ref>{{cite journal |
|||
|last=Barrer|first=R. M.|coauthors=Stuart, W. I. |
|||
|title=Non-Stoichiometric Clathrate of Water |
|||
|journal=Proceedings of the Royal Society of London |
|||
|year=1957|volume=243 |
|||
|issue=1233|pages=172–189 |
|||
|doi=10.1098/rspa.1957.0213|bibcode = 1957RSPSA.243..172B }}</ref> |
|||
Xenon can also form [[endohedral fullerene]] compounds, where a xenon atom is trapped inside a [[fullerene]] molecule. The xenon atom trapped in the fullerene can be monitored via <sup>129</sup>Xe [[nuclear magnetic resonance]] (NMR) spectroscopy. Using this technique, chemical reactions on the fullerene molecule can be analyzed, due to the sensitivity of the [[chemical shift]] of the xenon atom to its environment. However, the xenon atom also has an electronic influence on the reactivity of the fullerene.<ref>{{cite journal |
|||
==Adverse effects== |
|||
|last=Frunzi|first=Michael |
|||
Haloperidol is noted for its strong early and late [[extrapyramidal symptoms|extrapyramidal side effect]]s.<ref name="pmid17054159"/> The risk of the face-disfiguring [[tardive dyskinesia]] is around 4% per year in younger patients. Other predisposing factors may be female gender, pre-existing affective disorder, and cerebral dysfunction. [[Akathisia]] often manifests itself with anxiety, dysphoria, and an inability to remain motionless. |
|||
|coauthors=Cross, R. James; Saunders, Martin |
|||
|title=Effect of Xenon on Fullerene Reactions |
|||
|journal=Journal of the American Chemical Society |
|||
|year=2007 |
|||
|pmid=17924634|volume=129 |
|||
|doi=10.1021/ja075568n |
|||
|issue=43|page=13343 |
|||
|pages=13343–6}}</ref> |
|||
While xenon atoms are at their [[stationary state|ground energy state]], they repel each other and will not form a bond. When xenon atoms becomes energized, however, they can form an [[excimer]] (excited dimer) until the electrons return to the [[ground state]]. This entity is formed because the xenon atom tends to fill its outermost [[Electron shell|electronic shell]], and can briefly do this by adding an electron from a neighboring xenon atom. The typical lifetime of a xenon excimer is 1–5 ns, and the decay releases [[photon]]s with [[wavelength]]s of about 150 and 173 [[Nanometre|nm]].<ref>{{cite book |
|||
Other side effects include [[dry mouth]], [[lethargy]], restlessness of [[akathisia]], [[muscle]] stiffness or cramping, restlessness, [[tremor]]s, [[Rabbit syndrome]], and [[weight]] gain; side effects like these are more likely to occur when the drug is given in high doses and/or during long-term treatment. [[clinical depression|Depression]], severe enough to result in [[suicide]], is quite often seen during long-term treatment. Care should be taken to detect and treat depression early in course. Sometimes the change from haloperidol to a mildly potent neuroleptic (e.g., [[chlorprothixene]] or [[chlorpromazine]]), together with appropriate antidepressant therapy, does help. Sedative and anticholinergic side effects occur more frequently in the elderly. The likelihood of one's experiencing one or more of these side effects is quite high regardless of age and gender, especially with prolonged use. |
|||
|first=William Thomas|last=Silfvast |
|||
|year=2004|title=Laser Fundamentals |
|||
|publisher=Cambridge University Press |
|||
|isbn=0-521-83345-0|url=http://books.google.com/?id=x3VB2iwSaxsC&pg=RA1-PA152 |
|||
}}</ref><ref>{{cite book |
|||
|first=John G.|last=Webster|year=1998 |
|||
|title=The Measurement, Instrumentation, and Sensors Handbook |
|||
|publisher=Springer|isbn=3-540-64830-5 |
|||
|url=http://books.google.com/?id=b7UuZzf9ivIC&pg=PT2427}}</ref> Xenon can also form excimers with other elements, such as the [[halogen]]s [[bromine]], [[chlorine]] and [[fluorine]].<ref>{{cite book |
|||
|first=Charles|last=McGhee|year=1997 |
|||
|coauthors=Taylor, Hugh R.; Gartry, David S.; Trokel, Stephen L. |
|||
|title=Excimer Lasers in Ophthalmology |
|||
|publisher=Informa Health Care |
|||
|isbn=1-85317-253-7|url=http://books.google.com/?id=pg0bUc_GcVoC&pg=PA4}}</ref> |
|||
==Applications== |
|||
Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include spasm of the neck muscles sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first-generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups. |
|||
Although xenon is rare and relatively expensive to extract from the [[Earth's atmosphere]], it has a number of applications. |
|||
===Illumination and optics=== |
|||
The potentially fatal [[neuroleptic malignant syndrome]] (NMS) is a significant possible side effect. Haloperidol and [[fluphenazine]] cause NMS most often. NMS involves fever and other symptoms. Allergic and toxic side effects occur. Skin rash and photosensitivity both occur in fewer than 1% of patients. Children and adolescents are particularly sensitive to the early and late extrapyramidal side effects of haloperidol. It is not recommended to treat pediatric patients. |
|||
====Gas-discharge lamps==== |
|||
Xenon is used in light-emitting devices called xenon flash lamps, which are used in [[Flash (photography)|photographic flashes]] and stroboscopic lamps;<ref name="burke">{{cite book |
|||
|first=James|last=Burke|year=2003 |
|||
|title=Twin Tracks: The Unexpected Origins of the Modern World |
|||
|publisher=Oxford University Press |
|||
|isbn=0-7432-2619-4|page=33}}</ref> to excite the [[active laser medium|active medium]] in [[laser]]s which then generate [[coherent light]];<ref>{{cite web |
|||
|author=Staff|year=2007 |
|||
|url=http://www.praxair.com/praxair.nsf/1928438066cae92d85256a63004b880d/32f3a328e11bb600052565660052c139?OpenDocument |
|||
|title=Xenon Applications|publisher=Praxair Technology |
|||
|accessdate=2007-10-04}}</ref> and, occasionally, in [[Bactericide|bactericidal]] lamps.<ref>{{cite journal |
|||
|last=Baltás|first=E.|coauthors=Csoma, Z.; Bodai, L.; Ignácz, F.; Dobozy, A.; Kemény, L. |
|||
|title=A xenon-iodine electric discharge bactericidal lamp |
|||
|journal=Technical Physics Letters |
|||
|year=2003|volume=29|issue=10|pages=871–872 |
|||
|doi=10.1134/1.1623874|bibcode = 2003TePhL..29..871S }}</ref> The first solid-state [[laser]], invented in 1960, was pumped by a xenon flash lamp,<ref name="toyserkani">{{cite book |
|||
|last=Toyserkani|first=E.|year=2004 |
|||
|coauthors=Khajepour, A.; Corbin, S.|page=48 |
|||
|title=Laser Cladding|publisher=CRC Press |
|||
|isbn=0-8493-2172-7|url=http://books.google.com/?id=zfvbyCHzVqMC&pg=PA48}}</ref> and lasers used to power [[inertial confinement fusion]] are also pumped by xenon flash lamps.<ref>{{cite journal |
|||
|last=Skeldon|first=M.D. |
|||
|coauthors=Saager, R.; Okishev, A.; Seka, W. |
|||
|title=Thermal distortions in laser-diode- and flash-lamp-pumped Nd:YLF laser rods |
|||
|journal=LLE Review|year=1997|volume=71 |
|||
|pages=137–144|url=http://www.lle.rochester.edu/pub/review/v71/6_thermal.pdf |
|||
|accessdate=2007-02-04 |format=PDF| archiveurl = http://web.archive.org/web/20031016171340/http://www.lle.rochester.edu/pub/review/v71/6_thermal.pdf| archivedate = October 16, 2003}}</ref> |
|||
[[Image:Xenon short arc 1.jpg|thumb|Xenon short-arc lamp|alt=Elongated glass sphere with two metal rod electrodes inside, facing each other. One electrode is blunt and another is sharpened.]] |
|||
[[Long QT syndrome|QT prolongation]] with sudden death is a rarely seen, but clinically significant, side effect. Likewise, the development of [[thrombosis|thromboembolic]] complications are also seen. |
|||
[[Image:STS-135_Atlantis_rollout_1.jpg|thumb|Space Shuttle Atlantis bathed in xenon lights]] |
|||
[[Image:Xenon discharge tube.jpg|thumb|Xenon gas discharge tube]] |
|||
Continuous, short-arc, high pressure [[xenon arc lamp]]s have a [[color temperature]] closely approximating noon sunlight and are used in [[Solar Simulator|solar simulators]]. That is, the [[chromaticity]] of these lamps closely approximates a heated [[black body]] radiator that has a temperature close to that observed from the Sun. After they were first introduced during the 1940s, these lamps began replacing the shorter-lived [[carbon arc lamp]]s in movie projectors.<ref name="mellor">{{cite book |
|||
|first=David|last=Mellor|year=2000|page=186 |
|||
|title=Sound Person's Guide to Video |
|||
|publisher=Focal Press |
|||
|isbn=0-240-51595-1|url=http://books.google.com/?id=g93XXNA8Wf4C&pg=PA186}}</ref> They are employed in typical [[35 mm film|35mm]], [[IMAX]] and the new [[digital projectors]] [[Movie projector|film projection]] systems, automotive [[High-intensity discharge lamp|HID]] headlights, high-end [[tactical light|"tactical" flashlights]] and other specialized uses. These arc lamps are an excellent source of short wavelength [[ultraviolet]] radiation and they have intense emissions in the near [[infrared]], which is used in some [[night vision]] systems. |
|||
The individual cells in a [[plasma display]] use a mixture of xenon and neon that is converted into a [[plasma (physics)|plasma]] using [[electrode]]s. The interaction of this plasma with the electrodes generates ultraviolet [[photon]]s, which then excite the [[phosphor]] coating on the front of the display.<ref>{{cite web |
|||
Haloperidol may have a negative impact on vigilance or decrease the ability of the patient to drive or operate a machine, particularly initially. |
|||
|author=Anonymous |
|||
|url=http://www.plasmatvscience.org/theinnerworkings.html |
|||
|title=The plasma behind the plasma TV screen |
|||
|publisher=Plasma TV Science|accessdate=2007-10-14}}</ref><ref>{{cite news |
|||
|last=Marin|first=Rick|date=March 21, 2001 |
|||
|title=Plasma TV: That New Object Of Desire |
|||
|publisher=The New York Times|url=http://www.nytimes.com/2001/03/25/style/plasma-tv-that-new-object-of-desire.html?sec=&spon= |
|||
|accessdate=2009-04-03}}</ref> |
|||
Xenon is used as a "starter gas" in [[Sodium vapor lamp|high pressure sodium lamps]]. It has the lowest [[thermal conductivity]] and lowest [[ionization potential]] of all the non-radioactive noble gases. As a noble gas, it does not interfere with the chemical reactions occurring in the operating lamp. The low thermal conductivity minimizes thermal losses in the lamp while in the operating state, and the low ionization potential causes the [[breakdown voltage]] of the gas to be relatively low in the cold state, which allows the lamp to be more easily started.<ref>{{cite book |
|||
|first = John|last = Waymouth|year = 1971 |
|||
|title = Electric Discharge Lamps|publisher = The M.I.T. Press |
|||
|location = Cambridge, MA|isbn = 0-262-23048-8}}</ref> |
|||
====Lasers==== |
|||
==Other considerations== |
|||
In 1962, a group of researchers at [[Bell Labs|Bell Laboratories]] discovered laser action in xenon,<ref>{{cite journal |
|||
During long-term treatment of chronic psychiatric disorders, the daily dose should be reduced to the lowest level needed for maintenance of remission. Sometimes, it may be indicated to terminate haloperidol treatment gradually.<ref>{{cite web|url=http://www.chemeurope.com/en/encyclopedia/Haloperidol.html|title=Haloperidol at Chemeurope}}</ref> |
|||
|first=C. K. N.|last=Patel|coauthors=Bennett Jr., W. R.; Faust, W. L.; McFarlane, R. A. |
|||
|title=Infrared spectroscopy using stimulated emission techniques |
|||
|volume=9|issue=3|date=August 1, 1962|pages=102–104 |
|||
|journal=Physical Review Letters |
|||
|doi=10.1103/PhysRevLett.9.102 |bibcode=1962PhRvL...9..102P}}</ref> and later found that the laser gain was improved by adding [[helium]] to the lasing medium.<ref>{{cite journal |
|||
|first=C. K. N.|last=Patel |
|||
|coauthors=Faust, W. L.; McFarlane, R. A. |
|||
|title=High gain gaseous (Xe-He) optical masers |
|||
|journal=Applied Physics Letters |
|||
|volume=1|issue=4|pages=84–85|date=December 1, 1962 |
|||
|doi=10.1063/1.1753707 |
|||
|bibcode = 1962ApPhL...1...84P }}</ref><ref>{{cite journal |
|||
|first=W. R.|last=Bennett, Jr. |
|||
|title=Gaseous optical masers |
|||
|journal=Applied Optics Supplement |
|||
|volume=1|year=1962|pages=24–61}}</ref> The first [[excimer laser]] used a xenon [[Dimer (chemistry)|dimer]] (Xe<sub>2</sub>) energized by a beam of electrons to produce [[stimulated emission]] at an [[ultraviolet]] wavelength of 176 [[nanometre|nm]].<ref name="basov">{{cite journal |
|||
|doi=10.1070/QE1971v001n01ABEH003011 |
|||
|last=Basov|first=N. G. |
|||
|coauthors=Danilychev, V. A.; Popov, Yu. M. |
|||
|title=Stimulated Emission in the Vacuum Ultraviolet Region |
|||
|journal=Soviet Journal of Quantum Electronics |
|||
|year=1971|volume=1|issue=1|pages=18–22|bibcode = 1971QuEle...1...18B }}</ref> |
|||
Xenon chloride and xenon fluoride have also been used in excimer (or, more accurately, exciplex) lasers.<ref>{{cite web |
|||
|url=http://www.safetyoffice.uwaterloo.ca/hse/laser/documents/laser_types.html |
|||
|title=Laser Output|publisher=University of Waterloo |
|||
|accessdate=2007-10-07}}</ref> The xenon chloride excimer laser has been employed, for example, in certain dermatological uses.<ref>{{cite journal |
|||
|doi=10.1111/j.1468-3083.2006.01495.x |
|||
|first=E.|last=Baltás |
|||
|coauthors=Csoma, Z.; Bodai, L.; Ignácz, F.; Dobozy, A.; Kemény, L. |
|||
|title=Treatment of atopic dermatitis with the xenon chloride excimer laser |
|||
|journal=Journal of the European Academy of Dermatology and Venereology |
|||
|year=2006|volume=20|issue=6|pages=657–60 |
|||
|pmid=16836491}}</ref> |
|||
===Medical=== |
|||
Other forms of therapy (psychotherapy, occupational therapy/ergotherapy, or social rehabilitation) should be instituted properly. |
|||
====Anesthesia==== |
|||
Xenon has been used as a [[general anesthetic]]. Although it is expensive, anesthesia machines that can deliver xenon are about to appear on the European market, because advances in recovery and recycling of xenon have made it economically viable.<ref name="singh" /><ref>{{cite journal|last=Tonner|first=P. H.|title=Xenon: one small step for anaesthesia ... ? (editorial review)|journal=Current Opinion in Anaesthesiology|year=2006|volume=19|issue=4|pages=382–4|doi=10.1097/01.aco.0000236136.85356.13|pmid=16829718}}</ref> |
|||
Xenon affects many different receptors and neurotransmitters, like many theoretically multi-modal inhalation anesthetics they are likely complementary. Xenon is a high affinity glycine-site NMDA antagonist. However, xenon distinguishes itself from other clinically used NMDA antagonists in its lack of neurotoxicity and ability to inhibit the neurotoxicity of ketamine and nitrous oxide. Xenon does not provoke the release of dopamine from the nucleus accumbens and inhibits the meso-accumbal DA efflux from ketamine and nitrous oxide. Xenon activates the two-pore domain potassium channel TREK-1. Xenon inhibits nicotinic acetylcholine alpha4beta2 receptors which contribute to spinally mediated analgesia. |
|||
==Pregnancy and lactation== |
|||
Data from [[Animal testing|animal experiments]] indicate haloperidol is not [[teratogenic]], but is embryotoxic in high doses. In humans, no controlled studies exist. Unconfirmed studies in pregnant women revealed possible damage to the fetus, although most of the women were exposed to multiple drugs during pregnancy. Following accepted general principles, haloperidol should be given during pregnancy only if the benefit to the mother clearly outweighs the potential fetal risk.<ref>{{cite web|url=http://www.drugs.com/pro/haldol.html|title=Haldol at Drugs.com}}</ref> |
|||
Xenon is a competitive inhibitor of serotonin 5HT3. While neither anesthetic nor antinociceptive this activity reduces anesthesia-emergent nausea and vomiting. |
|||
Haloperidol, when given to lactating women, is found in significant amounts in their milk. Breastfed children sometimes show extrapyramidal symptoms. If the use of haloperidol during lactation seems indicated, the benefit for the mother should clearly outweigh the risk for the child, or breastfeeding should be stopped. |
|||
== Carcinogenicity == |
|||
In an unconfirmed study at the Buffalo Psychiatric Center, relative risks of breast cancer in inmates undergoing long-term treatment with haloperidol were 3.5 times higher than those of patients at the general hospital, and 9.5 times higher than the reported incidents in the general population.<ref name="pmid8599407">{{cite journal |author=Halbreich U, Shen J, Panaro V |title=Are chronic psychiatric patients at increased risk for developing breast cancer? |journal=Am J Psychiatry |volume=153 |issue=4 |pages=559–60 |year=1996 |pmid=8599407 |doi= |url=http://ajp.psychiatryonline.org/cgi/pmidlookup?view=long&pmid=8599407}}</ref> These results need confirmation by larger studies, and so far, no statistically acceptable evidence has been found to associate long-term use of haloperidol with the potential for increased breast cancer risk in female patients. |
|||
Two physiological mechanisms for xenon anesthesia have been proposed. The first one involves the inhibition of the [[Plasma membrane Ca2+ ATPase|calcium ATPase pump]]—the mechanism cells use to remove calcium (Ca<sup>2+</sup>)—in the [[cell membrane]] of [[Chemical synapse|synapses]].<ref name=Franks1995>{{Cite journal|last=Franks|first=JJ|coauthors=Horn JL, Janicki PK, Singh G|title=Halothane, isoflurane, xenon, and nitrous oxide inhibit calcium ATPase pump activity in rat brain synaptic plasma membranes|journal=Anesthesiology|volume=82|issue=1|pages=108–17|year=1995|pmid=7832292|doi=10.1097/00000542-199501000-00015|url=http://journals.lww.com/anesthesiology/fulltext/1995/01000/halothane,_isoflurane,_xenon,_and_nitrous_oxide.15.aspx#|accessdate=2010-09-15}}</ref> This results from a [[conformational isomerism|conformational change]] when xenon binds to nonpolar sites inside the protein.<ref name=Lopez1995>{{Cite journal|last=Lopez|first=MM|coauthors=Kosk-Kosicka D|title=How do volatile anesthetics inhibit Ca<sub>2</sub><sup>+</sup>-ATPases?|journal=Journal of Biological Chemistry|volume=270|issue=47|pages=28239–45|year=1995|pmid=7499320|doi=10.1074/jbc.270.47.28239|url=http://www.jbc.org/content/270/47/28239.full|accessdate=2010-09-15}}</ref> |
|||
==Neurotoxic metabolites== |
|||
Haloperidol has been shown to metabolize in murine<ref>{{cite journal|title=Studies on the metabolism of haloperidol (HP): the role of CYP3A in the production of the neurotoxic pyridinium metabolite HPP+ found in rat brain following ip administration of HP.|journal=Life Sci. 1995 Nov 17;57(26):2439-46.|pmid=8847965|url=http://www.ncbi.nlm.nih.gov/pubmed/8847965}}</ref>, and human<ref>{{cite journal|title=Studies on the conversion of haloperidol and its tetrahydropyridine dehydration product to potentially neurotoxic pyridinium metabolites by human liver microsomes.|journal=Chem Res Toxicol. 1996 Jun;9(4):800-|pmid=8831826|url=http://www.ncbi.nlm.nih.gov/pubmed/8831826}}</ref> hepatocytes via CYP-3A4 to the neurotoxic pyridinium metabolites 4-(4-chlorophenyl)-1-(4-fluorophenyl)-4-oxobutylpyridinium(HPP+)and 4-(4-chlorophenyl)-1-(4-fluorophenyl)-4-hydroxybutylpyridinium (RHPP+).<ref>{{cite journal|title=Cytochrome P450-mediated metabolism of haloperidol and reduced haloperidol to pyridinium metabolites.|journal=Chem Res Toxicol. 2006 Jul;19(7):914-20.|pmid=16841959|url=http://www.ncbi.nlm.nih.gov/pubmed/16841959}}</ref> HPP+ and RHPP+ are lipophilic and have elimination half lives of 67.3 hrs and 63.3 hrs, respectively.<ref>{{cite journal|title=Metabolism of haloperidol to pyridinium species in patients receiving high doses intravenously: is HPTP an intermediate?|journal=Life Sci. 1997;61(24):2383-90.|pmid=9399630|url=http://www.ncbi.nlm.nih.gov/pubmed/9399630}}</ref> HPP+ is a structural analog of the more widely known Parkinson’s producing neurotoxin [[MPP+]] and its precursor [[MPTP]]. Unlike MPP+, HPP+ is not dependent on MAO-B for metabolism to toxic species and does not require functional dopamine transporter protein for intracellular uptake.<ref>{{cite journal|title=Binding of 4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]pyridinium ion (HPP+), a metabolite of haloperidol, to synthetic melanin: implications for the dopaminergic neurotoxicity of HPP+.|journal=Neurotox Res. 2004;6(7-8):535-42.|pmid=15639785|url=http://www.ncbi.nlm.nih.gov/pubmed/15639785}}</ref> |
|||
Microdialysis studies were performed in the striatum, substantia nigra and cortex of conscious rats to compare the neurotoxic potential of 1-methyl-4-phenylpyridinium (MPP+) and HPP+ to dopaminergic and serotonergic neurons. HPP+ was a less potent neurotoxin than MPP+ to dopaminergic neurons and displayed equipotent serotonergic neurotoxicity.<ref>{{cite journal|title=MPP(+)-like neurotoxicity of a pyridinium metabolite derived from haloperidol: in vivo microdialysis and in vitro mitochondrial studies.|journal=J Pharmacol Exp Ther. 1994 Jan;268(1):380-7|pmid=8301579|url=http://www.ncbi.nlm.nih.gov/pubmed/8301579}}</ref> Impairment of cortico-striatal mitochondrial complex I is pathognomic of MPP+ neurotoxicity and parkinson’s cellular dysfunction.<ref>{{cite journal|title=Mitochondrial complex I inhibition in Parkinson's disease: how can curcumin protect mitochondria?|journal=Antioxid Redox Signal. 2007 Mar;9(3):399-408.|pmid=17184173|url=http://www.ncbi.nlm.nih.gov/pubmed/17184173}}</ref><ref>{{cite journal|title=Mitochondria, oxidative damage, and inflammation in Parkinson's disease.|journal=Ann N Y Acad Sci. 2003 Jun;991:120-31.|pmid=12846981|url=http://www.ncbi.nlm.nih.gov/pubmed/12846981}}</ref> HPP+ is more potent than MPP+ at inhibiting murine mitochondrial complex I with an IC50 of 12mMol for HPP+ and 160mMol for MPP+.<ref>{{cite journal|title=MPP(+)-like neurotoxicity of a pyridinium metabolite derived from haloperidol: in vivo microdialysis and in vitro mitochondrial studies.|journal=J Pharmacol Exp Ther. 1994 Jan;268(1):380-7|pmid=8301579|url=http://www.ncbi.nlm.nih.gov/pubmed/8301579}}</ref> Prolonged, high dose (2 & 5mg\kg) administration of haloperidol in a murine model elevates striatal nitric oxide, [[TNF-a]], and [[caspase-3]].<ref>{{cite journal|title=Activation of striatal inflammatory mediators and caspase-3 is central to haloperidol-induced orofacial dyskinesia.|journal=Eur J Pharmacol. 2008 Aug 20;590(1-3):241-5. Epub 2008 Jun 14|pmid=18590723|url=http://www.ncbi.nlm.nih.gov/pubmed/18590723}}</ref> |
|||
HPP+ and RHPP+ have been found in the brains of patients taking Haldol at autopsy.<ref>{{cite journal|title=Two pyridinium metabolites of haloperidol are present in the brain of patients at post-mortem.|journal=Life Sci. 1997;60(8):529-34.|pmid=9042387|url=http://www.ncbi.nlm.nih.gov/pubmed/9042387}}</ref> A short term 6 week trial failed to find statistically significant correlation between HPP+, RHPP+ and extrapyramidal symptoms.<ref>{{cite journal|title=Disposition of haloperidol pyridinium and reduced haloperidol pyridinium in schizophrenic patients: no relationship with clinical variables during short-term treatment.|journal=J Clin Psychopharmacol. 2000 Apr;20(2):210-9.|pmid=10770460|url=http://www.ncbi.nlm.nih.gov/pubmed/10770460}}</ref> A long term retrospective study found a significant positive correlation between levels of HPP+ and severity of tardive dyskinesia.<ref>{{cite journal|title=Serum concentrations of haloperidol pyridinium metabolites and the relationship with tardive dyskinesia and parkinsonism: a cross-section study in psychiatric patients.|journal=Pharmacopsychiatry. 2005 Jul;38(4):171-7|url=http://www.ncbi.nlm.nih.gov/pubmed/16025420}}</ref> |
|||
Xenon has a [[minimum alveolar concentration]] (MAC) of 72% at age 40, making it 44% more potent than N<sub>2</sub>O as an anesthetic.<ref>{{Cite journal|last=Nickalls |first=R. W. D|coauthors=Mapleson, W.W.|title=Age‐related iso‐MAC charts for isoflurane, sevoflurane and desflurane in man|journal= British Journal of Anesthesiology|url=http://bja.oxfordjournals.org/content/91/2/170/T1.expansion.html}}</ref> Thus it can be used in concentrations with oxygen that have a lower risk of [[Hypoxia (medical)|hypoxia]]. Unlike [[nitrous oxide]] (N<sub>2</sub>O), xenon is not a [[greenhouse gas]] and so it is also viewed as [[environmentally friendly]].<ref name=Goto2003>{{Cite journal|last=Goto|first=T|coauthors=Nakata Y, Morita S|title=Will xenon be a stranger or a friend?: the cost, benefit, and future of xenon anesthesia|journal=Anesthesiology|volume=98|issue=1|pages=1–2|year=2003|pmid=12502969|url=http://journals.lww.com/anesthesiology/Fulltext/2003/01000/Will_Xenon_Be_a_Stranger_or_a_Friend___The_Cost,.2.aspx|accessdate=2010-09-15|doi=10.1097/00000542-200301000-00002}}</ref> Xenon vented into the atmosphere is being returned to its original source, so no environmental impact is likely. |
|||
==Interactions== |
|||
* Other central depressants (alcohol, tranquilizers, narcotics): actions and side effects of these drugs (sedation, respiratory depression) are increased. In particular, the doses of concomitantly used opioids for chronic pain can be reduced by 50%. |
|||
* [[Methyldopa]]: increased risk of extrapyramidal side effects and other unwanted central effects |
|||
* [[Levodopa]]: decreased action of levodopa |
|||
* [[Tricyclic antidepressants]]: metabolism and elimination of tricyclics significantly decreased, increased toxicity noted (anticholinergic and cardiovascular side effects, lowering of seizure threshold) |
|||
* [[Quinidine]], [[buspirone]], and [[fluoxetine]]: increased plasma levels of haloperidol, decrease haloperidol dose, if necessary |
|||
* [[Carbamazepine]], [[phenobarbital]], and [[rifampicin]]: plasma levels of haloperidol significantly decreased, increase haloperidol dose, if necessary |
|||
* [[Lithium salt|lithium]]: rare cases of the following symptoms have been noted: [[encephalopathy]], early and late extrapyramidal side effects, other neurologic symptoms, and coma.<ref>{{cite web|url=http://www.ncbi.nlm.nih.gov/pubmed/6415823|title=Toxic irreversible encephalopathy induced by lithium carbonate and haloperidol. A report of 2 cases.}}</ref> |
|||
* [[Guanethidine]]: antihypertensive action antagonized |
|||
* [[Epinephrine]]: action antagonized, paradoxical decrease in blood pressure may result |
|||
* [[Amphetamine]] and [[methylphenidate]]: counteracts increased action of norepinephrine and dopamine in patients with [[narcolepsy]] or [[ADD]]/[[ADHD]] |
|||
====Neuroprotectant==== |
|||
==Doses== |
|||
Xenon holds great promise as a rapid-acting, low toxicity, high potency neuroprotectant. Xenon is a high affinity antagonist at the NMDA receptor glycine site. |
|||
As directed by the physician, the dose needed depends on the condition to be treated, age, and weight of patient: |
|||
Xenon is cardioprotective in ischemia-reperfusion conditions by inducing pharmacologic non-ischemic preconditioning. Xenon is cardioprotective by activating PKC-epsilon & downstream p38-MAPK. http://www.ncbi.nlm.nih.gov/pubmed/15644876 |
|||
* Acute problems: single doses of 1 to 5 mg (up to 10 mg) oral or IM, usually repeated every four to six hours, not exceeding an oral dose of 100 mg daily. Doses used for IV injection are usually five to 10 mg as a single dose; not exceeding 50 mg daily. The British National Formulary recommends a maximum daily dose of 30 mg total (IM and oral) with a maximum of 18 mg by the IM route. |
|||
Xenon mimics neuronal ischemic preconditioning by activating ATP sensitive potassium channels. http://www.ncbi.nlm.nih.gov/pubmed/19352153 Xenon allosterically reduces ATP mediated channel activation inhibition independently of the sulfonylurea receptor1 subunit, increasing KATP open-channel time and frequency. http://www.ncbi.nlm.nih.gov/pubmed/20179498 |
|||
Xenon upregulates hypoxia inducible factor 1 alpha (HIF1a). |
|||
http://www.isrn.com/journals/anesthesiology/2011/510297/ |
|||
Xenon gas was added as an ingredient of the [[Mechanical ventilation|ventilation mix]] for a newborn baby at [[St. Michael's Hospital, Bristol]], England, whose life chances were otherwise very compromised, and was successful, leading to the authorisation of [[clinical trial]]s for similar cases.<ref>{{cite news | author=Staff |
|||
:PET imaging studies have suggested low doses are preferable. Clinical response was associated with at least 65% occupancy of D2 receptors, while greater than 72% was likely to cause hyperprolactinaemia and over 78% associated with extrapyramidal side effects. Doses of haloperidol greater than 5 mg increased the risk of side effects without improving efficacy.<ref>P Oosthuizen, RA Emsley, J Turner et al. Determining the optimal dose of haloperidol in first-episode psychosis. Journal of Psychopharmacology. 2001, 15: 251–255.</ref> Patients responded with doses under even 2 mg in first episode psychosis.<ref>J Tauscher, S Kapur. CNS Drugs. 2001, 15, 9: 671–678(8)</ref> |
|||
| title=First baby given xenon gas to prevent brain injury |
|||
* Chronic conditions: 0.5 to 20 mg daily oral doses are recommended, rarely more. The lowest dose that maintains remission is employed. |
|||
| work=BBC News | date=April 9, 2010 |
|||
* Experimental doses: In resistant cases of psychosis, small studies with oral doses of up to 300 to 500 mg daily have been conducted (in most cases with an anticholinergic, anti-Parkinsonian drug (Biperiden, Benzatropine, etc.) to avoid severe early extrapyramidal side effects. These studies showed no superior results and led to severe side effects. Also, the frequency of otherwise unusual side effects (hypotension, QT-time prolongation, and serious [[cardiac arrhythmia]]s) was dramatically increased. The clinical use of haloperidol in these doses is discouraged now and it is recommended to switch the patient gradually to a different neuroleptic (e.g., clozapine, olanzapine, aripiprazole). |
|||
| url=http://news.bbc.co.uk/1/hi/england/bristol/8611130.stm |
|||
| accessdate=2010-04-09 }}</ref> The treatment is done simultaneously with [[Hypothermia therapy for neonatal encephalopathy|cooling]] the [[Thermoregulation|body temperature]] to {{nowrap|33.5 °C}}.<ref>{{cite web | accessdate=2011-10-19 |
|||
| first=Sian | last=Newman | publisher=Swansea University |
|||
| url=http://www.swan.ac.uk/medicine/newsandevents/ilsnewsbulletinhiddenpages/drjohndingley-babyriley/ |
|||
| title=Xenon gas used in a bid to reduce brain injury in newborns }}</ref> |
|||
====Imaging==== |
|||
*Depot forms are also available; these are injected deeply IM at regular intervals. The depot forms are not suitable for initial treatment, but are suitable for patients who have demonstrated inconsistency with oral dosages. |
|||
[[gamma ray|Gamma]] emission from the [[radioisotope]] <sup>133</sup>Xe of xenon can be used to image the heart, lungs, and brain, for example, by means of [[single photon emission computed tomography]]. <sup>133</sup>Xe has also been used to measure [[blood flow]].<ref>{{cite book |
|||
|first=Ernst|last=Van Der Wall|year=1992 |
|||
|title=What's New in Cardiac Imaging?: SPECT, PET, and MRI |
|||
|publisher=Springer|isbn=0-7923-1615-0|url=http://books.google.com/?id=PypZMUhqnK8C&pg=PA41 |
|||
}}</ref><ref>{{cite journal |
|||
|last=Frank|first=John |
|||
|title=Introduction to imaging: The chest |
|||
|journal=Student BMJ|year=1999|volume=12|pages=1–44 |
|||
|url=http://student.bmj.com/issues/04/01/education/8.php |
|||
|accessdate=2008-06-04 |
|||
}}</ref><ref>{{cite web |
|||
|last=Chandak|first=Puneet K.|date=July 20, 1995 |
|||
|url=http://brighamrad.harvard.edu/education/online/BrainSPECT/Theory/Xenon133.html |
|||
|title=Brain SPECT: Xenon-133|publisher=Brigham RAD |
|||
|accessdate=2008-06-04}}</ref> |
|||
Xenon, particularly hyperpolarized <sup>129</sup>Xe, is a useful contrast agent for [[MRI|magnetic resonance imaging]] (MRI). In the gas phase, it can be used to image empty space such as cavities in a porous sample or alveoli in lungs. [[Hyperpolarization (physics)|Hyperpolarization]] renders <sup>129</sup>Xe much more detectable via [[magnetic resonance imaging]] and has been used for studies of the lungs and other tissues. It can be used, for example, to trace the flow of gases within the lungs.<ref>{{cite journal |
|||
==Overdose== |
|||
|last=Albert|first=M. S.|coauthors=Balamore, D. |
|||
Experimental evidence from animal studies indicates the doses needed for acute poisoning are quite high in relation to therapeutic doses. Overdoses with depot injections are uncommon, because only certified personnel are legally permitted to administer them to patients. |
|||
|title=Development of hyperpolarized noble gas MRI |
|||
|journal=Nuclear Instruments and Methods in Physics Research A |
|||
|year=1998|volume=402 |
|||
|issue=2–3|pages=441–53 |
|||
|doi=10.1016/S0168-9002(97)00888-7 |pmid=11543065 |
|||
|bibcode = 1998NIMPA.402..441A }}</ref><ref>{{cite news |
|||
|last=Irion|first=Robert|date=March 23, 1999 |
|||
|title=Head Full of Xenon?|publisher=Science News |
|||
|url=http://sciencenow.sciencemag.org/cgi/content/full/1999/323/3 |
|||
|accessdate=2007-10-08| archiveurl = http://web.archive.org/web/20040117194538/http://sciencenow.sciencemag.org/cgi/content/full/1999/323/3| archivedate = January 17, 2004}}</ref> Because xenon is soluble in water and also in hydrophobic solvents, it can be used to image various soft living tissues.<ref>{{cite journal |
|||
|title=Intravascular delivery of hyperpolarized 129Xenon for in vivo MRI |
|||
|journal= Applied Magnetic Resonance |volume=15|issue=3–4 |
|||
|year=1998|doi=10.1007/BF03162020 |
|||
|pages=343–352|author=Wolber, J. |
|||
|last2=Rowland |
|||
|first2=I. J. |
|||
|last3=Leach |
|||
|first3=M. O. |
|||
|last4=Bifone |
|||
|first4=A.}}</ref><ref>{{cite journal |
|||
|pmid=19703880 |year=2009 |author=Driehuys, B.; Möller, H.E.; Cleveland, Z.I.; Pollaro, J.; Hedlund, L.W.; |
|||
|title=Pulmonary perfusion and xenon gas exchange in rats: MR imaging with intravenous injection of hyperpolarized 129Xe |
|||
|volume=252 |pages=386–93 |
|||
|doi=10.1148/radiol.2522081550|pmc=2753782 |
|||
|journal=Radiology |
|||
|issue=2|ssrn=2}}</ref><ref>{{cite journal |
|||
|pmid=19702286|year=2009 |
|||
|author=Cleveland, Z.I.; Möller, H.E.; Hedlund, L.W.; Driehuys, B. |
|||
|title=Continuously infusing hyperpolarized 129Xe into flowing aqueous solutions using hydrophobic gas exchange membranes |
|||
|volume=113 |issue=37 |pages=12489–99 |
|||
|doi=10.1021/jp9049582 |pmc=2747043 |
|||
|journal=The journal of physical chemistry}}</ref> |
|||
=== |
===NMR spectroscopy=== |
||
Because of the atom's large, flexible outer electron shell, the [[nuclear magnetic resonance|NMR]] spectrum changes in response to surrounding conditions, and can therefore be used as a probe to measure the chemical circumstances around the xenon atom. For instance xenon dissolved in water, xenon dissolved in hydrophobic solvent, and xenon associated with certain proteins can be distinguished by NMR.<ref>{{cite journal |
|||
Symptoms are usually due to exaggerated side effects. Most often encountered are: |
|||
|journal=Magnetic Resonance in Chemistry |volume=27 |
|||
|issue=10 |page=950 |doi=10.1002/mrc.1260271009 |
|||
|title=Interpretation of the solvent effect on the screening constant of Xe-129 |author=Luhmer, M. |
|||
|year=1989 |
|||
|last2=Dejaegere |
|||
|first2=A. |
|||
|last3=Reisse |
|||
|first3=J. |
|||
}}</ref><ref>{{cite journal |
|||
|author=Rubin, Seth M.; Spence, Megan M.; Goodson, Boyd M.; Wemmer, David E.; Pines, Alexander |
|||
|title=Evidence of nonspecific surface interactions between laser-polarized xenon and myoglobin in solution |
|||
|journal=Proceedings of the National Academy of Science USA |
|||
|date=August 15, 2000 |volume=97 |
|||
|pmid=10931956 |issue=17 |
|||
|pmc=16888 |pages=9472–5 |
|||
|doi=10.1073/pnas.170278897|bibcode = 2000PNAS...97.9472R }}</ref> |
|||
Hyperpolarized xenon can be used by [[Surface science|surface-chemists]]. Normally, it is difficult to characterize surfaces using NMR, because signals from the surface of a sample will be overwhelmed by signals from the far-more-numerous atomic nuclei in the bulk. However, nuclear spins on solid surfaces can be selectively polarized, by [[Proton Enhanced Nuclear Induction Spectroscopy|transferrering spin polarization to them]] from hyperpolarized xenon gas. This makes the surface signals strong enough to measure, and distinguishes them from bulk signals.<ref>{{cite journal |
|||
* Severe [[extrapyramidal]] side effects with muscle rigidity and tremors, [[akathisia]], etc. |
|||
|doi=10.1021/ja972035d |title=Optical Pumping and Magic Angle Spinning: Sensitivity and Resolution Enhancement for Surface NMR Obtained with Laser-Polarized Xenon |
|||
* [[Hypotension]] or [[hypertension]] |
|||
|year=1997 |author=Raftery, Daniel; MacNamara, Ernesto; Fisher, Gregory; Rice, Charles V.; Smith, Jay |
|||
* [[Sedation]] |
|||
|journal=Journal of the American Chemical Society |
|||
* [[Anticholinergic]] side effects (dry mouth, constipation, paralytic [[ileus]], difficulties in urinating, decreased [[perspiration]]) |
|||
|volume=119 |
|||
* Coma in severe cases, accompanied by respiratory depression and massive hypotension, shock |
|||
|issue=37 |page=8746 |
|||
* Rarely, serious ventricular arrhythmia (''[[torsades de pointes]]''), with or without prolonged [[QT-time]] |
|||
}}</ref><ref>{{cite journal |
|||
* Epileptic seizures |
|||
|author=Gaede, H. C.; Song, Y. -Q.; Taylor, R. E.; Munson, E. J.; Reimer, J. A.; Pines, A. |
|||
|doi=10.1007/BF03162652 |
|||
|title=High-field cross polarization NMR from laser-polarized xenon to surface nuclei |
|||
|year=1995 |journal=Applied Magnetic Resonance |
|||
|volume=8 |
|||
|issue=3–4 |page=373}}</ref> |
|||
=== |
===Other=== |
||
In [[Nuclear physics|nuclear energy]] applications, xenon is used in [[bubble chamber]]s,<ref>{{cite book |
|||
Treatment is merely symptomatic and involves intensive care with stabilization of vital functions. In early detected cases of oral overdose, induction of [[emesis]], [[gastric lavage]], and the use of [[activated charcoal]] can all be tried. [[Epinephrine]] is avoided for treatment of hypotension and shock, because its action might be reversed. In the case of a severe overdose, antidotes such as [[bromocryptine]] or [[ropinirole]] may be used to treat the extrapyramidal effects caused by haloperidol, acting as dopamine receptor agonists. |
|||
|first=Peter Louis|last=Galison|year=1997 |
|||
|title=Image and Logic: A Material Culture of Microphysics |
|||
|page=339|url=http://books.google.com/?id=HnRDiDtO5yoC&pg=PA339|publisher=University of Chicago Press|isbn=0-226-27917-0}}</ref> probes, and in other areas where a high [[Molecular mass|molecular weight]] and inert nature is desirable. A by-product of [[nuclear weapon]] testing is the release of radioactive xenon-133 and xenon-135. The detection of these isotopes is used to monitor compliance with nuclear |
|||
[[Test Ban Treaty (disambiguation)|test ban treaties]],<ref>{{cite journal |
|||
|author=Fontaine, J.-P.; Pointurier, F.; Blanchard, X.; Taffary, T.|title=Atmospheric xenon radioactive isotope monitoring|journal=Journal of Environmental Radioactivity |
|||
|volume=72|issue=1–2|pages=129–35|year=2004 |
|||
|doi=10.1016/S0265-931X(03)00194-2 |
|||
|pmid=15162864}}</ref> as well as |
|||
to confirm nuclear test explosions by states such as [[North Korea]].<ref>{{cite journal |
|||
|author=Garwin, Richard L.; von Hippel Frank N. |
|||
|title=A Technical Analysis: Deconstructing North Korea's October 9 Nuclear Test|publisher=Arms Control Association |
|||
|journal=Arms Control Today|volume=38|issue=9 |
|||
|month=November|year=2006|accessdate=2009-03-26 |
|||
|url=http://www.armscontrol.org/act/2006_11/tech}}</ref> |
|||
[[Image:Xenon ion engine prototype.png|left|thumb|A prototype of a xenon ion engine being tested at NASA's [[Jet Propulsion Laboratory]].|alt=A metal cylinder with electrodes attached to its side. Blue diffuse light is coming out of the tube.]] |
|||
===Prognosis=== |
|||
Liquid xenon is being used in [[Calorimeter (particle physics)|calorimeters]]<ref>{{cite journal |
|||
In general, the prognosis of overdose is good, and lasting damage is not known, provided the patient has survived the initial phase. An overdose of haloperidol can be fatal.<ref>{{cite web|url=http://www.drugs.com/mtm/haloperidol.html|title=Haloperidol at Drugs.com}}</ref> |
|||
|author=Gallucci, G. |
|||
|title=The MEG liquid xenon calorimeter |
|||
|journal=Journal of Physics: Conference Series |
|||
|volume=160 |
|||
|issue=1 |
|||
|year=2009 |
|||
|doi=10.1088/1742-6596/160/1/012011 |
|||
|page=012011 |
|||
|bibcode = 2009JPhCS.160a2011G }}</ref> for measurements of [[gamma ray]]s as well as a medium for detecting hypothetical [[weakly interacting massive particles]], or WIMPs. When a WIMP collides with a xenon nucleus, it should, theoretically, strip an electron and create a primary [[Scintillation (physics)|scintillation]]. By using xenon, this burst of energy could then be readily distinguished from similar events caused by particles such as [[cosmic ray]]s.<ref name="ball">{{cite web |
|||
|last=Ball|first=Philip|date=May 1, 2002 |
|||
|url=http://www.nature.com/news/2002/020429/full/news020429-6.html |
|||
|title=Xenon outs WIMPs|publisher=Nature |
|||
|accessdate=2007-10-08}}</ref> However, the XENON experiment at the [[Gran Sasso National Laboratory]] in Italy and the ZEPLIN-II and ZEPLIN-III experiments at the [[Boulby Underground Laboratory]] in the UK have thus far failed to find any confirmed WIMPs. Even if no WIMPs are detected, the experiments will serve to constrain the properties of [[dark matter]] and some physics models.<ref>{{cite web |
|||
|last=Schumann|first=Marc|date=October 10, 2007 |
|||
|url=http://xenon.physics.rice.edu/ |
|||
|title=XENON announced new best limits on Dark Matter |
|||
|publisher=Rice University|accessdate=2007-10-08 |
|||
}}</ref><ref>{{cite journal | display-authors=1 |
|||
| last1=Lebedenko | first1=V. N. | last2=Araújo | first2=H. M. |
|||
| last3=Barnes | first3=E. J. | last4=Bewick | first4=A. |
|||
| last5=Cashmore | first5=R. | last6=Chepel | first6=V. |
|||
| last7=Currie | first7=A. | last8=Davidge | first8=D. |
|||
| last9=Dawson | first9=J. |
|||
|title=Results from the first science run of the ZEPLIN-III dark matter search experiment |
|||
|journal=Physical Review D|volume=80|issue=5 |
|||
|year=2009|doi=10.1103/PhysRevD.80.052010 |
|||
|bibcode=2009PhRvD..80e2010L |
|||
|page=052010 }}</ref> The current detector at the Gran Sasso facility has demonstrated sensitivity comparable to that of the best cryogenic detectors, and the sensitivity was expected to be increased by an [[order of magnitude]] in 2009.<ref>{{cite news |
|||
|last=Boyd|first=Jade|date=August 23, 2007 |
|||
|title=Rice physicists go deep for 'dark matter' |
|||
|publisher=Hubble News Desk |
|||
|url=http://www.media.rice.edu/media/NewsBot.asp?MODE=VIEW&ID=9902&SnID=1256234278 |
|||
|accessdate=2007-10-08}}</ref> |
|||
Xenon is the preferred [[propellant]] for [[ion propulsion]] of [[spacecraft]] because of its low [[ionization potential]] per [[Atomic mass|atomic weight]], and its ability to be stored as a liquid at near [[room temperature]] (under high pressure) yet be easily converted back into a gas to feed the engine. The inert nature of xenon makes it environmentally friendly and less corrosive to an [[ion engine]] than other fuels such as [[Mercury (element)|mercury]] or [[caesium]]. Xenon was first used for satellite ion engines during the 1970s.<ref>{{cite web |
|||
==Other formulations== |
|||
|last=Zona|first=Kathleen|date=March 17, 2006 |
|||
[[Image:Haloperidol decanoate highlighting ester group.svg|thumb|[[Skeletal formula]] of haloperidol decanoate: The decanoate group is highlighted in {{color|blue|blue}}.]] |
|||
|url=http://www.nasa.gov/centers/glenn/about/fs08grc.html |
|||
The [[decanoate]] ester of haloperidol (haloperidol decanoate, trade names Haldol decanoate, Halomonth, Neoperidole) has a much longer duration of action, so is often used in people known to be noncompliant with oral medication. A dose of 25 to 250 mg is given by intramuscular injection once every two to four weeks.<ref>Goodman and Gilman's ''Pharmacological Basis of Therapeutics, 10th edition'' (McGraw-Hill, 2001).</ref> |
|||
|title=Innovative Engines: Glenn Ion Propulsion Research Tames the Challenges of 21st century Space Travel |
|||
|publisher=NASA|accessdate=2007-10-04}}</ref> It was later employed as a propellant for JPL's [[Deep Space 1]] probe, Europe's [[SMART-1]] spacecraft<ref name="saccoccia">{{cite news |
|||
|last=Saccoccia|first=G. |
|||
|coauthors=del Amo, J. G.; Estublier, D. |
|||
|title=Ion engine gets SMART-1 to the Moon |
|||
|date=August 31, 2006|publisher=ESA |
|||
|url=http://www.esa.int/SPECIALS/SMART-1/SEMLZ36LARE_0.html|accessdate=2007-10-01}}</ref> and for the three ion propulsion engines on NASA's [[Dawn Spacecraft]].<ref>{{cite web |
|||
|url=http://www.jpl.nasa.gov/news/press_kits/dawn-launch.pdf|format=PDF |
|||
|title=Dawn Launch: Mission to Vesta and Ceres |
|||
|publisher=NASA|accessdate=2007-10-01}}</ref> |
|||
Chemically, the [[perxenate]] compounds are used as [[oxidizing agent]]s in [[analytical chemistry]]. [[Xenon difluoride]] is used as an etchant for [[silicon]], particularly in the production of [[microelectromechanical systems]] (MEMS).<ref>{{cite conference |
|||
The [[IUPAC name]] of haloperidol decanoate is 4-(4-chlorophenyl)-1-1[4-(4-fluorophenyl)-4-oxobutyl]-4 piperidinyl decanoate. |
|||
|last=Brazzle|first=J. D. |
|||
|coauthors=Dokmeci, M. R.; Mastrangelo, C. H. |
|||
|title=Modeling and Characterization of Sacrificial Polysilicon Etching Using Vapor-Phase Xenon Difluoride |
|||
|booktitle=Proceedings 17th IEEE International Conference on Micro Electro Mechanical Systems (MEMS) |
|||
|pages=737–740 |
|||
|publisher=IEEE |
|||
|date=July 28 – August 1, 1975 |
|||
|location=Maastricht, Netherlands |
|||
|isbn=978-0-7803-8265-7}}</ref> The anticancer drug [[Fluorouracil|5-fluorouracil]] can be produced by reacting xenon difluoride with [[uracil]].<ref> |
|||
{{cite web |
|||
|author=Staff|year=2007 |
|||
|url=http://portal.acs.org/portal/PublicWebSite/education/whatischemistry/landmarks/bartlettnoblegases/index.htm |
|||
|title=Neil Bartlett and the Reactive Noble Gases|publisher=American Chemical Society |
|||
|accessdate=June 5, 2012}}</ref> Xenon is also used in [[X-ray crystallography|protein crystallography]].</ref> Applied at pressures from 0.5 to 5 [[Pascal (unit)|MPa]] (5 to 50 [[atmosphere (unit)|atm]]) to a protein crystal, xenon atoms bind in predominantly [[Hydrophobe|hydrophobic]] cavities, often creating a high quality, isomorphous, heavy-atom derivative, which can be used for solving the [[phase problem]].<ref>{{cite web |
|||
|author=Staff|date=December 21, 2004 |
|||
|url=http://www.srs.ac.uk/px/facilities/xenon_notes_1.html |
|||
|archiveurl=http://web.archive.org/web/20050316174727/http://www.srs.ac.uk/px/facilities/xenon_notes_1.html |
|||
|archivedate=2005-03-16 |
|||
|title=Protein Crystallography: Xenon and Krypton Derivatives for Phasing |
|||
|publisher=Daresbury Laboratory, PX |
|||
|accessdate=2007-10-01 |
|||
}}</ref><ref>{{cite book |
|||
|first=Jan|last=Drenth|coauthors=Mesters, Jeroen |
|||
|chapter=The Solution of the Phase Problem by the Isomorphous Replacement Method |
|||
|pages=123–171|doi=10.1007/0-387-33746-6_7 |
|||
|title=Principles of Protein X-Ray Crystallography |
|||
|publisher=Springer|location=New York |
|||
|isbn= 978-0-387-33334-2|edition=3rd |
|||
|year=2007}}</ref> |
|||
<div style="clear:both;"></div> |
|||
== |
==Precautions== |
||
Many oxygen-containing [[xenon compounds]] are toxic due to their strong [[oxidation|oxidative]] properties, and explosive due to their tendency to break down into elemental xenon plus diatomic oxygen (O<sub>2</sub>), which contains much stronger chemical bonds than the xenon compounds.<ref name="finkel68">{{cite web |
|||
Haloperidol is also used on many different kinds of animals. It appears to be particularly successful when given to birds, e.g., a parrot that will otherwise continuously pluck its feathers out.<ref>{{cite web|url=http://www.lcrx.com/veterinary_avian.html|title=Veterinary:Avian at Lloyd Center Pharmacy}}</ref> |
|||
|last=Finkel|first=A. J. |
|||
|coauthors=Katz, J. J.; Miller, C. E. |
|||
|date=April 1, 1968 |
|||
|url=http://ntrs.nasa.gov/search.jsp?R=306918&id=2&qs=No%3D40%26Ne%3D26%26N%3D297%2B140%26Ns%3DPublicationYear%257C0 |
|||
|title=Metabolic and toxicological effects of water-soluble xenon compounds are studied |
|||
|publisher=NASA|accessdate=2007-10-04}}</ref> |
|||
Xenon gas can be safely kept in normal sealed glass or metal containers at [[standard temperature and pressure]]. However, it readily dissolves in most plastics and rubber, and will gradually escape from a container sealed with such materials.<ref>{{ |
|||
==Dose forms== |
|||
cite journal |
|||
* Liquid: 2.0 and 10 mg/ml |
|||
|last=LeBlanc|first=Adrian D. |
|||
* Tablets: 0.5, 1.0, 2.0, 5.0, 10, and 20 mg |
|||
|coauthors=Johnson, Philip C. |
|||
* Injection: 5 mg (1 ml) |
|||
|title=The handling of xenon-133 in clinical studies |
|||
* Depot injection forms |
|||
|year=1971|journal=Physics in Medicine and Biology |
|||
* The original brand Haldol and many generics are available |
|||
|volume=16|issue=1|pages=105–9 |
|||
|doi=10.1088/0031-9155/16/1/310 |
|||
|pmid=5579743|bibcode = 1971PMB....16..105L }}</ref> Xenon is non-[[toxic]], although it does dissolve in blood and belongs to a select group of substances that penetrate the [[blood–brain barrier]], causing mild to full surgical [[anesthesia]] when inhaled in high concentrations with oxygen.<ref name="finkel68" /> |
|||
At 169 m/s, the [[speed of sound]] in xenon gas is slower than that in air<ref>169.44 m/s in xenon (at 0°C and 107 KPa), compared to 344 m/s in air. See: {{cite journal |
|||
|last=Vacek|first=V. |
|||
|coauthors=Hallewell, G.; Lindsay, S. |
|||
|title=Velocity of sound measurements in gaseous per-fluorocarbons and their mixtures |
|||
|journal=Fluid Phase Equilibria |
|||
|year=2001|volume=185 |
|||
|issue=1–2|pages=305–314 |
|||
|doi = 10.1016/S0378-3812(01)00479-4}}</ref> due to the slower average speed of the heavy xenon atoms compared to nitrogen and oxygen molecules. Hence, xenon lowers the resonant frequencies of the [[vocal tract]] when inhaled. This produces a characteristic lowered voice timbre, an effect opposite to the high-timbred voice caused by inhalation of [[helium]]. Like helium, xenon does not satisfy the body's need for oxygen. Xenon is both a simple [[asphyxiant gas|asphyxiant]] and an anesthetic more powerful than nitrous oxide; consequently, many universities no longer allow the voice stunt as a general chemistry demonstration. As xenon is expensive, the gas [[sulfur hexafluoride]], which is similar to xenon in molecular weight (146 versus 131), is generally used in this stunt, and is an asphyxiant without being anesthetic.<ref>{{cite web |
|||
|first=Steve|last=Spangler|year=2007 |
|||
|url=http://www.stevespanglerscience.com/experiment/from-donald-duck-to-barry-white-how-gases-change-your-voice |
|||
|title=Anti-Helium – Sulfur Hexafluoride |
|||
|publisher=Steve Spangler Science |
|||
|accessdate=2007-10-04}}</ref> |
|||
It is possible to safely breathe heavy gases such as xenon or sulfur hexafluoride when they are in a mixture with oxygen; the oxygen comprising at least 20% of the mixture. Xenon at 80% concentration along with 20% oxygen rapidly produces the unconsciousness of general anesthesia (and has been used for this, as discussed above). Breathing mixes gases of different densities very effectively and rapidly so that heavier gases are purged along with the oxygen, and do not accumulate at the bottom of the lungs.<ref>{{cite journal |
|||
|last=Yamaguchi|first=K. |
|||
|coauthors=Soejima, K.; Koda, E.; Sugiyama, N |
|||
|title=Inhaling Gas With Different CT Densities Allows Detection of Abnormalities in the Lung Periphery of Patients With Smoking-Induced COPD |
|||
|journal=Chest Journal |
|||
|year=2001|volume=51|pages=1907–16 |
|||
|doi= 10.1378/chest.120.6.1907|pmid=11742921|issue=6}}</ref> There is, however, a danger associated with any heavy gas in large quantities: it may sit invisibly in a container, and if a person enters a container filled with an odorless, colorless gas, they may find themselves breathing it unknowingly. Xenon is rarely used in large enough quantities for this to be a concern, though the potential for danger exists any time a tank or container of xenon is kept in an unventilated space.<ref>{{cite web |
|||
|author=Staff|date=August 1, 2007 |
|||
|url=http://www-group.slac.stanford.edu/esh/hazardous_substances/cryogenic/p_hazards.htm |
|||
|title=Cryogenic and Oxygen Deficiency Hazard Safety |
|||
|publisher=Stanford Linear Accelerator Center |
|||
|accessdate=2007-10-10| archiveurl = http://web.archive.org/web/20070609173316/http://www-group.slac.stanford.edu/esh/hazardous_substances/cryogenic/p_hazards.htm| archivedate = June 9, 2007}}</ref> |
|||
==See also== |
==See also== |
||
* |
*[[Buoyant levitation]] |
||
*[[Penning mixture]] |
|||
{{Subject bar |
|||
|book1=Xenon |
|||
|book2=Period 5 elements |
|||
|book3=Noble gases |
|||
|book4=Chemical elements (sorted alphabetically) |
|||
|book5=Chemical elements (sorted by number) |
|||
|portal=Chemistry |
|||
|commons=y |
|||
|wikt=y |
|||
|wikt-search=xenon |
|||
|v=y |
|||
|v-search=Xenon atom |
|||
}} |
|||
==References== |
==References== |
||
{{ |
{{reflist|35em}} |
||
==External links== |
==External links== |
||
* |
*[http://www.webelements.com/webelements/elements/text/Xe/index.html WebElements.com – Xenon] |
||
* |
*[http://wwwrcamnl.wr.usgs.gov/isoig/period/xe_iig.html USGS Periodic Table – Xenon] |
||
*[http://environmentalchemistry.com/yogi/periodic/Xe.html EnvironmentalChemistry.com – Xenon] |
|||
* [http://www.kompendium.ch/MonographieTxt.aspx?lang=de&MonType=fi Swiss scientific information on Haldol] |
|||
*[http://www.anaesthetist.com/anaes/drugs/xenon.htm Xenon as an anesthetic] |
|||
*{{cite web |
|||
*[http://nobelprize.org/nobel_prizes/chemistry/laureates/1904/ramsay-lecture.html Sir William Ramsay's Nobel-Prize lecture (1904)] |
|||
| url = http://www.who.int/medicines/publications/essentialmedicines/Updated_sixteenth_adult_list_en.pdf |
|||
| title = WHO Model List of Essential Medicines |
|||
{{compact periodic table}} |
|||
| edition = 16th list (updated) |
|||
{{Xenon compounds}} |
|||
| publisher = World Health Organization |
|||
{{featured article}} |
|||
| accessdate = 2010-09-14 |
|||
| format = PDF |
|||
| month = March |
|||
| year = 2010 |
|||
}} |
|||
* [http://druginfo.nlm.nih.gov/drugportal/dpdirect.jsp?name=Haloperidol U.S. National Library of Medicine: Drug Information Portal - Haloperidol] |
|||
[[Category:Xenon]] |
|||
{{Antipsychotics}} |
|||
[[Category:Noble gases]] |
|||
{{Antiemetics}} |
|||
[[Category:Chemical elements]] |
|||
{{Dopaminergics}} |
|||
[[Category:General anesthetics]] |
|||
{{Adrenergics}} |
|||
[[Category:NMDA receptor antagonists]] |
|||
{{Serotonergics}} |
|||
{{Cholinergics}} |
|||
{{Histaminergics}} |
|||
{{Link FA|sv}} |
|||
{{Link FA|de}} |
|||
<!-- Don't categorize user sandbox pages into main article categories --> |
|||
<!-- interwiki --> |
|||
[[:Category:Antiemetics]] |
|||
[[:Category:Organofluorides]] |
|||
[[:Category:Organochlorides]] |
|||
[[:Category:Alcohols]] |
|||
[[:Category:Piperidines]] |
|||
[[:Category:Butyrophenone antipsychotics]] |
|||
[[:Category:Janssen Pharmaceutica]] |
|||
[[:Category:Belgian inventions]] |
|||
[[ |
[[af:Xenon]] |
||
[[ |
[[ar:زينون]] |
||
[[ |
[[an:Xenón]] |
||
[[ |
[[gn:Tatapytagua]] |
||
[[az:Ksenon]] |
|||
[[el:Αλοπεριδόλη]] |
|||
[[ |
[[bn:জেনন]] |
||
[[be:Ксенон]] |
|||
[[fa:هالوپریدول]] |
|||
[[be-x-old:Ксэнон]] |
|||
[[fr:Halopéridol]] |
|||
[[ |
[[bg:Ксенон]] |
||
[[ |
[[bs:Ksenon]] |
||
[[ |
[[ca:Xenó]] |
||
[[ |
[[cv:Ксенон]] |
||
[[cs:Xenon]] |
|||
[[ja:ハロペリドール]] |
|||
[[ |
[[co:Xenu]] |
||
[[ |
[[cy:Senon]] |
||
[[ |
[[da:Xenon]] |
||
[[ |
[[de:Xenon]] |
||
[[et:Ksenoon]] |
|||
[[ru:Галоперидол]] |
|||
[[ |
[[el:Ξένο]] |
||
[[ |
[[es:Xenón]] |
||
[[ |
[[eo:Ksenono]] |
||
[[ |
[[eu:Xenon]] |
||
[[fa:زنون]] |
|||
[[hif:Xenon]] |
|||
[[fr:Xénon]] |
|||
[[fur:Xenon]] |
|||
[[ga:Xeanón]] |
|||
[[gv:Xenon]] |
|||
[[gl:Xenon]] |
|||
[[xal:Ксенөн]] |
|||
[[ko:제논 (원소)]] |
|||
[[hy:Քսենոն]] |
|||
[[hi:ज़ेनान]] |
|||
[[hr:Ksenon]] |
|||
[[io:Xenono]] |
|||
[[id:Xenon]] |
|||
[[ia:Xenon]] |
|||
[[is:Xenon]] |
|||
[[it:Xeno]] |
|||
[[he:קסנון]] |
|||
[[jv:Xenon]] |
|||
[[kn:ಝೆನಾನ್]] |
|||
[[ka:ქსენონი]] |
|||
[[kk:Ксенон]] |
|||
[[sw:Xenoni]] |
|||
[[kv:Ксенон]] |
|||
[[mrj:Ксенон]] |
|||
[[la:Xenon]] |
|||
[[lv:Ksenons]] |
|||
[[lb:Xenon]] |
|||
[[lt:Ksenonas]] |
|||
[[lij:Seno]] |
|||
[[li:Xenon]] |
|||
[[jbo:fangynavni]] |
|||
[[hu:Xenon]] |
|||
[[mk:Ксенон]] |
|||
[[ml:സെനൊൺ]] |
|||
[[mr:झेनॉन]] |
|||
[[ms:Xenon]] |
|||
[[my:ဇီနွန်]] |
|||
[[nl:Xenon]] |
|||
[[ja:キセノン]] |
|||
[[no:Xenon]] |
|||
[[nn:Xenon]] |
|||
[[oc:Xenon]] |
|||
[[uz:Ksenon]] |
|||
[[nds:Xenon]] |
|||
[[pl:Ksenon]] |
|||
[[pt:Xenônio]] |
|||
[[ro:Xenon]] |
|||
[[qu:Senun]] |
|||
[[ru:Ксенон]] |
|||
[[stq:Xenon]] |
|||
[[scn:Xenu]] |
|||
[[simple:Xenon]] |
|||
[[sk:Xenón]] |
|||
[[sl:Ksenon]] |
|||
[[sr:Ксенон]] |
|||
[[sh:Ksenon]] |
|||
[[fi:Ksenon]] |
|||
[[sv:Xenon]] |
|||
[[tl:Henon (elemento)]] |
|||
[[ta:செனான்]] |
|||
[[th:ซีนอน]] |
|||
[[tr:Ksenon]] |
|||
[[uk:Ксенон]] |
|||
[[ur:زینون]] |
|||
[[ug:كسېنون]] |
|||
[[vep:Ksenon]] |
|||
[[vi:Xenon]] |
|||
[[war:Xenon]] |
|||
[[yi:קסענאן]] |
|||
[[yo:Ksenon]] |
|||
[[zh-yue:氙]] |
|||
[[zh:氙]] |
|||
Revision as of 01:38, 12 August 2012
 A xenon-filled discharge tube glowing light blue | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xenon | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | colorless gas, exhibiting a blue glow when placed in an electric field | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Xe) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xenon in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 54 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 18 (noble gases) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | p-block | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 4d10 5s2 5p6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 18, 8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | gas | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 161.40 K (−111.75 °C, −169.15 °F) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 165.051 K (−108.099 °C, −162.578 °F) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density when solid (at t.p.) | 3.408 g/cm3[5] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (at STP) | 5.894 g/L | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at b.p.) | 2.942 g/cm3[6] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Triple point | 161.405 K, 81.77 kPa[7] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Critical point | 289.733 K, 5.842 MPa[7] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 2.27 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 12.64 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 21.01[8] J/(mol·K) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | common: +2, +4, +6 0,[9] +8[10] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.60 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 140±9 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 216 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc) (cF4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lattice constant | a = 634.84 pm (at triple point, 161.405 K)[5] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 5.65×10−3 W/(m⋅K) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[11] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −43.9×10−6 cm3/mol (298 K)[12] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | gas: 178 m·s−1 liquid: 1090 m/s | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-63-3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery and first isolation | William Ramsay and Morris Travers (1898) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of xenon | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Xenon (/[invalid input: 'icon']ˈzɛnɒn/ ZEN-on or /ˈziːnɒn/ ZEE-non) is a chemical element with the symbol Xe and atomic number 54. It is a colorless, heavy, odorless noble gas, that occurs in the Earth's atmosphere in trace amounts.[17] Although generally unreactive, xenon can undergo a few chemical reactions such as the formation of xenon hexafluoroplatinate, the first noble gas compound to be synthesized.[18][19][20]
Naturally occurring xenon consists of eight stable isotopes. There are also over 40 unstable isotopes that undergo radioactive decay. The isotope ratios of xenon are an important tool for studying the early history of the Solar System.[21] Radioactive xenon-135 is produced from iodine-135 as a result of nuclear fission, and it acts as the most significant neutron absorber in nuclear reactors.[22]
Xenon is used in flash lamps[23] and arc lamps,[24] and as a general anesthetic.[25] The first excimer laser design used a xenon dimer molecule (Xe2) as its lasing medium,[26] and the earliest laser designs used xenon flash lamps as pumps.[27] Xenon is also being used to search for hypothetical weakly interacting massive particles[28] and as the propellant for ion thrusters in spacecraft.[29]
History
Xenon was discovered in England by the Scottish chemist William Ramsay and English chemist Morris Travers on July 12, 1898, shortly after their discovery of the elements krypton and neon. They found Xenon in the residue left over from evaporating components of liquid air.[30][31] Ramsay suggested the name xenon for this gas from the Greek word ξένον [xenon], neuter singular form of ξένος [xenos], meaning 'foreign(er)', 'strange(r)', or 'guest'.[32][33] In 1902, Ramsay estimated the proportion of xenon in the Earth's atmosphere as one part in 20 million.[34]
During the 1930s, American engineer Harold Edgerton began exploring strobe light technology for high speed photography. This led him to the invention of the xenon flash lamp, in which light is generated by sending a brief electrical current through a tube filled with xenon gas. In 1934, Edgerton was able to generate flashes as brief as one microsecond with this method.[23][35][36]
In 1939, American physician Albert R. Behnke Jr. began exploring the causes of "drunkenness" in deep-sea divers. He tested the effects of varying the breathing mixtures on his subjects, and discovered that this caused the divers to perceive a change in depth. From his results, he deduced that xenon gas could serve as an anesthetic. Although Russian toxicologist Nikolay V. Lazarev apparently studied xenon anesthesia in 1941, the first published report confirming xenon anesthesia was in 1946 by American medical researcher John H. Lawrence, who experimented on mice. Xenon was first used as a surgical anesthetic in 1951 by American anesthesiologist Stuart C. Cullen, who successfully operated on two patients.[37]
Xenon and the other noble gases were for a long time considered to be completely chemically inert and not able to form compounds. However, while teaching at the University of British Columbia, Neil Bartlett discovered that the gas platinum hexafluoride (PtF6) was a powerful oxidizing agent that could oxidize oxygen gas (O2) to form dioxygenyl hexafluoroplatinate (O2+[PtF6]–).[38] Since O2 and xenon have almost the same first ionization potential, Bartlett realized that platinum hexafluoride might also be able to oxidize xenon. On March 23, 1962, he mixed the two gases and produced the first known compound of a noble gas, xenon hexafluoroplatinate.[39][20] Bartlett thought its composition to be Xe+[PtF6]–, although later work has revealed that it was probably a mixture of various xenon-containing salts.[40][41][42] Since then, many other xenon compounds have been discovered,[43] along with some compounds of the noble gases argon, krypton, and radon, including argon fluorohydride (HArF),[44] krypton difluoride (KrF2),[45][46] and radon fluoride.[47] By 1971, more than 80 xenon compounds were known.[48][49]
Characteristics

(animated version)
Xenon has atomic number 54; that is, its nucleus contains 54 protons. At standard temperature and pressure, pure xenon gas has a density of 5.761 kg/m3, about 4.5 times the surface density of the Earth's atmosphere, 1.217 kg/m3.[50] As a liquid, xenon has a density of up to 3.100 g/mL, with the density maximum occurring at the triple point.[51] Under the same conditions, the density of solid xenon, 3.640 g/cm3, is higher than the average density of granite, 2.75 g/cm3.[51] Using gigapascals of pressure, xenon has been forced into a metallic phase.[52]
Solid xenon changes from face-centered cubic (fcc) to hexagonal close packed (hcp) crystal phase under pressure and begins to turn metallic at about 140 GPa, with no noticeable volume change in the hcp phase. It is completely metallic at 155 GPa. When metalized, xenon looks sky blue because it absorbs red light and transmits other visible frequencies. Such behavior is unusual for a metal and is explained by the relatively small widths of the electron bands in metallic xenon.[53][54]
Xenon is a member of the zero-valence elements that are called noble or inert gases. It is inert to most common chemical reactions (such as combustion, for example) because the outer valence shell contains eight electrons. This produces a stable, minimum energy configuration in which the outer electrons are tightly bound.[55] However, xenon can be oxidized by powerful oxidizing agents, and many xenon compounds have been synthesized.
In a gas-filled tube, xenon emits a blue or lavenderish glow when the gas is excited by electrical discharge. Xenon emits a band of emission lines that span the visual spectrum,[56] but the most intense lines occur in the region of blue light, which produces the coloration.[57]
Occurrence and production
Xenon is a trace gas in Earth's atmosphere, occurring at 87±1 parts per billion (nL/L), or approximately 1 part per 11.5 million,[58] and is also found in gases emitted from some mineral springs.
Xenon is obtained commercially as a byproduct of the separation of air into oxygen and nitrogen. After this separation, generally performed by fractional distillation in a double-column plant, the liquid oxygen produced will contain small quantities of krypton and xenon. By additional fractional distillation steps, the liquid oxygen may be enriched to contain 0.1–0.2% of a krypton/xenon mixture, which is extracted either via adsorption onto silica gel or by distillation. Finally, the krypton/xenon mixture may be separated into krypton and xenon via distillation.[59][60] Extraction of a liter of xenon from the atmosphere requires 220 watt-hours of energy.[61] Worldwide production of xenon in 1998 was estimated at 5,000–7,000 m3.[62] Because of its low abundance, xenon is much more expensive than the lighter noble gases—approximate prices for the purchase of small quantities in Europe in 1999 were 10 €/L for xenon, 1 €/L for krypton, and 0.20 €/L for neon.[62]
Within the Solar System, the nucleon fraction of xenon is 1.56 × 10−8, for an abundance of one part in 64 million of the total mass.[63] Xenon is relatively rare in the Sun's atmosphere, on Earth, and in asteroids and comets. The planet Jupiter has an unusually high abundance of xenon in its atmosphere; about 2.6 times as much as the Sun.[64] This high abundance remains unexplained and may have been caused by an early and rapid buildup of planetesimals—small, subplanetary bodies—before the presolar disk began to heat up.[65] (Otherwise, xenon would not have been trapped in the planetesimal ices.) The problem of the low terrestrial xenon may potentially be explained by covalent bonding of xenon to oxygen within quartz, hence reducing the outgassing of xenon into the atmosphere.[66]
Unlike the lower mass noble gases, the normal stellar nucleosynthesis process inside a star does not form xenon. Elements more massive than iron-56 have a net energy cost to produce through fusion, so there is no energy gain for a star when creating xenon.[67] Instead, xenon is formed during supernova explosions,[68] by the slow neutron capture process (s-process) of red giant stars that have exhausted the hydrogen at their cores and entered the asymptotic giant branch,[69] in classical nova explosions[70] and from the radioactive decay of elements such as iodine, uranium and plutonium.[71]
Isotopes and isotopic studies
Naturally occurring xenon is made of eight stable isotopes, the most of any element with the exception of tin, which has ten. Xenon and tin are the only elements to have more than seven stable isotopes.[72] The isotopes 124Xe and 134Xe are predicted to undergo double beta decay, but this has never been observed so they are considered to be stable.[73] Besides these stable forms, there are over 40 unstable isotopes that have been studied. The longest lived these isotopes is 136Xe, which has been observed to undergo double beta decay with a half-life of 2.11 x 1021yr.[74] 129Xe is produced by beta decay of 129I, which has a half-life of 16 million years, while 131mXe, 133Xe, 133mXe, and 135Xe are some of the fission products of both 235U and 239Pu,[71] and therefore used as indicators of nuclear explosions.
Nuclei of two of the stable isotopes of xenon, 129Xe and 131Xe, have non-zero intrinsic angular momenta (nuclear spins, suitable for nuclear magnetic resonance). The nuclear spins can be aligned beyond ordinary polarization levels by means of circularly polarized light and rubidium vapor.[75] The resulting spin polarization of xenon nuclei can surpass 50% of its maximum possible value, greatly exceeding the equilibrium value dictated by the Boltzmann distribution (typically 0.001% of the maximum value at room temperature, even in the strongest magnets). Such non-equilibrium alignment of spins is a temporary condition, and is called hyperpolarization. The process of hyperpolarizing the xenon is called optical pumping (although the process is different from pumping a laser).[76]
Because a 129Xe nucleus has a spin of 1/2, and therefore a zero electric quadrupole moment, the 129Xe nucleus does not experience any quadrupolar interactions during collisions with other atoms, and thus its hyperpolarization can be maintained for long periods of time even after the laser beam has been turned off and the alkali vapor removed by condensation on a room-temperature surface. Spin polarization of 129Xe can persist from several seconds for xenon atoms dissolved in blood[77] to several hours in the gas phase[78] and several days in deeply frozen solid xenon.[79] In contrast, 131Xe has a nuclear spin value of 3/2 and a nonzero quadrupole moment, and has T1 relaxation times in the millisecond and second ranges.[80]
Some radioactive isotopes of xenon, for example, 133Xe and 135Xe, are produced by neutron irradiation of fissionable material within nuclear reactors.[18] 135Xe is of considerable significance in the operation of nuclear fission reactors. 135Xe has a huge cross section for thermal neutrons, 2.6×106 barns,[22] so it acts as a neutron absorber or "poison" that can slow or stop the chain reaction after a period of operation. This was discovered in the earliest nuclear reactors built by the American Manhattan Project for plutonium production. Fortunately the designers had made provisions in the design to increase the reactor's reactivity (the number of neutrons per fission that go on to fission other atoms of nuclear fuel).[81] 135Xe reactor poisoning played a major role in the Chernobyl disaster.[82] A shutdown or decrease of power of a reactor can result in buildup of 135Xe and getting the reactor into the iodine pit.
Under adverse conditions, relatively high concentrations of radioactive xenon isotopes may be found emanating from nuclear reactors due to the release of fission products from cracked fuel rods,[83] or fissioning of uranium in cooling water.[84]
Because xenon is a tracer for two parent isotopes, xenon isotope ratios in meteorites are a powerful tool for studying the formation of the solar system. The iodine-xenon method of dating gives the time elapsed between nucleosynthesis and the condensation of a solid object from the solar nebula. In 1960, physicist John H. Reynolds discovered that certain meteorites contained an isotopic anomaly in the form of an overabundance of xenon-129. He inferred that this was a decay product of radioactive iodine-129. This isotope is produced slowly by cosmic ray spallation and nuclear fission, but is produced in quantity only in supernova explosions. As the half-life of 129I is comparatively short on a cosmological time scale, only 16 million years, this demonstrated that only a short time had passed between the supernova and the time the meteorites had solidified and trapped the 129I. These two events (supernova and solidification of gas cloud) were inferred to have happened during the early history of the Solar System, as the 129I isotope was likely generated before the Solar System was formed, but not long before, and seeded the solar gas cloud with isotopes from a second source. This supernova source may also have caused collapse of the solar gas cloud.[85][86]
In a similar way, xenon isotopic ratios such as 129Xe/130Xe and 136Xe/130Xe are also a powerful tool for understanding planetary differentiation and early outgassing.[21] For example, The atmosphere of Mars shows a xenon abundance similar to that of Earth: 0.08 parts per million,[87] however Mars shows a higher proportion of 129Xe than the Earth or the Sun. As this isotope is generated by radioactive decay, the result may indicate that Mars lost most of its primordial atmosphere, possibly within the first 100 million years after the planet was formed.[88][89] In another example, excess 129Xe found in carbon dioxide well gases from New Mexico was believed to be from the decay of mantle-derived gases soon after Earth's formation.[71][90]
Compounds
After Neil Bartlett's discovery in 1962 that xenon can form chemical compounds, a large number of xenon compounds have been discovered and described. Almost all known xenon compounds contain the electronegative atoms fluorine or oxygen.[91]
Halides
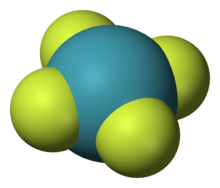

Three fluorides are known: XeF
2, XeF
4, and XeF
6. XeF is theorized to be unstable.[92] The fluorides are the starting point for the synthesis of almost all xenon compounds.
The solid, crystalline difluoride XeF
2 is formed when a mixture of fluorine and xenon gases is exposed to ultraviolet light.[93] Ordinary daylight is sufficient.[94] Long-term heating of XeF
2 at high temperatures under an NiF
2 catalyst yields XeF
6.[95] Pyrolysis of XeF
6 in the presence of NaF yields high-purity XeF
4.[96]
The xenon fluorides behave as both fluoride acceptors and fluoride donors, forming salts that contain such cations as XeF+
and Xe2F3+, and anions such as XeF5−, XeF7−, and XeF82−. The green, paramagnetic Xe2+ is formed by the reduction of XeF
2 by xenon gas.[91]
XeF
2 is also able to form coordination complexes with transition metal ions. Over 30 such complexes have been synthesized and characterized.[95]
Whereas the xenon fluorides are well-characterized, the other halides are not known, the only exception being the dichloride, XeCl2. Xenon dichloride is reported to be an endothermic, colorless, crystalline compound that decomposes into the elements at 80°C, formed by the high-frequency irradiation of a mixture of xenon, fluorine, and silicon or carbon tetrachloride.[97] However, doubt has been raised as to whether XeCl
2 is a real compound and not merely a van der Waals molecule consisting of weakly bound Xe atoms and Cl
2 molecules.[98] Theoretical calculations indicate that the linear molecule XeCl
2 is less stable than the van der Waals complex.[99]
Oxides and oxohalides
Three oxides of xenon are known: xenon trioxide (XeO
3) and xenon tetroxide (XeO
4), both of which are dangerously explosive and powerful oxidizing agents, and xenon dioxide (XeO2), which was reported in 2011 with a coordination number of four.[100] XeO2 forms when xenon tetrafluoride is poured over ice. Its crystal structure may allow it to replace silicon in silicate minerals.[101] The XeOO+ cation has been identified by infrared spectroscopy in solid argon.[102]
Xenon does not react with oxygen directly; the trioxide is formed by the hydrolysis of XeF
6:[103]
- XeF
6 + 3 H
2O → XeO
3 + 6 HF
XeO
3 is weakly acidic, dissolving in alkali to form unstable xenate salts containing the HXeO−
4 anion. These unstable salts easily disproportionate into xenon gas and perxenate salts, containing the XeO4−
6 anion.[104]
Barium perxenate, when treated with concentrated sulfuric acid, yields gaseous xenon tetroxide:[97]
- Ba
2XeO
6 + 2 H
2SO
4 → 2 BaSO
4 + 2 H
2O + XeO
4
To prevent decomposition, the xenon tetroxide thus formed is quickly cooled to form a pale-yellow solid. It explodes above −35.9 °C into xenon and oxygen gas.
A number of xenon oxyfluorides are known, including XeOF
2, XeOF
4, XeO
2F
2, and XeO
3F
2. XeOF
2 is formed by the reaction of OF
2 with xenon gas at low temperatures. It may also be obtained by the partial hydrolysis of XeF
4. It disproportionates at −20 °C into XeF
2 and XeO
2F
2.[105] XeOF
4 is formed by the partial hydrolysis of XeF
6,[106] or the reaction of XeF
6 with sodium perxenate, Na
4XeO
6. The latter reaction also produces a small amount of XeO
3F
2. XeOF
4 reacts with CsF to form the XeOF−
5 anion,[105][107] while XeOF3 reacts with the alkali metal fluorides KF, RbF and CsF to form the XeOF−
4 anion.[108]
Other compounds
Recently, there has been an interest in xenon compounds where xenon is directly bonded to a less electronegative element than fluorine or oxygen, particularly carbon.[109] Electron-withdrawing groups, such as groups with fluorine substitution, are necessary to stabilize these compounds.[104] Numerous such compounds have been characterized, including:[105][110]
- C
6F
5–Xe+
–N≡C–CH
3, where C6F5 is the pentafluorophenyl group. - [C
6F
5]
2Xe - C
6F
5–Xe–X, where X is CN, F, or Cl. - R–C≡C–Xe+
, where R is C
2F−
5 or tert-butyl. - C
6F
5–XeF+
2 - (C
6F
5Xe)
2Cl+
Other compounds containing xenon bonded to a less electronegative element include F–Xe–N(SO
2F)
2 and F–Xe–BF
2. The latter is synthesized from dioxygenyl tetrafluoroborate, O
2BF
4, at −100 °C.[105][111]
An unusual ion containing xenon is the tetraxenonogold(II) cation, AuXe2+
4, which contains Xe–Au bonds.[112] This ion occurs in the compound AuXe
4(Sb
2F
11)
2, and is remarkable in having direct chemical bonds between two notoriously unreactive atoms, xenon and gold, with xenon acting as a transition metal ligand.
In 1995, M. Räsänen and co-workers, scientists at the University of Helsinki in Finland, announced the preparation of xenon dihydride (HXeH), and later xenon hydride-hydroxide (HXeOH), hydroxenoacetylene (HXeCCH), and other Xe-containing molecules.[113] In 2008, Khriachtchev et al. reported the preparation of HXeOXeH by the photolysis of water within a cryogenic xenon matrix.[114] Deuterated molecules, HXeOD and DXeOH, have also been produced.[115]
Clathrates and excimers
In addition to compounds where xenon forms a chemical bond, xenon can form clathrates—substances where xenon atoms are trapped by the crystalline lattice of another compound. An example is xenon hydrate (Xe·5.75 H2O), where xenon atoms occupy vacancies in a lattice of water molecules.[116] This clathrate has a melting point of 24 °C.[117] The deuterated version of this hydrate has also been produced.[118] Such clathrate hydrates can occur naturally under conditions of high pressure, such as in Lake Vostok underneath the Antarctic ice sheet.[119] Clathrate formation can be used to fractionally distill xenon, argon and krypton.[120]
Xenon can also form endohedral fullerene compounds, where a xenon atom is trapped inside a fullerene molecule. The xenon atom trapped in the fullerene can be monitored via 129Xe nuclear magnetic resonance (NMR) spectroscopy. Using this technique, chemical reactions on the fullerene molecule can be analyzed, due to the sensitivity of the chemical shift of the xenon atom to its environment. However, the xenon atom also has an electronic influence on the reactivity of the fullerene.[121]
While xenon atoms are at their ground energy state, they repel each other and will not form a bond. When xenon atoms becomes energized, however, they can form an excimer (excited dimer) until the electrons return to the ground state. This entity is formed because the xenon atom tends to fill its outermost electronic shell, and can briefly do this by adding an electron from a neighboring xenon atom. The typical lifetime of a xenon excimer is 1–5 ns, and the decay releases photons with wavelengths of about 150 and 173 nm.[122][123] Xenon can also form excimers with other elements, such as the halogens bromine, chlorine and fluorine.[124]
Applications
Although xenon is rare and relatively expensive to extract from the Earth's atmosphere, it has a number of applications.
Illumination and optics
Gas-discharge lamps
Xenon is used in light-emitting devices called xenon flash lamps, which are used in photographic flashes and stroboscopic lamps;[23] to excite the active medium in lasers which then generate coherent light;[125] and, occasionally, in bactericidal lamps.[126] The first solid-state laser, invented in 1960, was pumped by a xenon flash lamp,[27] and lasers used to power inertial confinement fusion are also pumped by xenon flash lamps.[127]



Continuous, short-arc, high pressure xenon arc lamps have a color temperature closely approximating noon sunlight and are used in solar simulators. That is, the chromaticity of these lamps closely approximates a heated black body radiator that has a temperature close to that observed from the Sun. After they were first introduced during the 1940s, these lamps began replacing the shorter-lived carbon arc lamps in movie projectors.[24] They are employed in typical 35mm, IMAX and the new digital projectors film projection systems, automotive HID headlights, high-end "tactical" flashlights and other specialized uses. These arc lamps are an excellent source of short wavelength ultraviolet radiation and they have intense emissions in the near infrared, which is used in some night vision systems.
The individual cells in a plasma display use a mixture of xenon and neon that is converted into a plasma using electrodes. The interaction of this plasma with the electrodes generates ultraviolet photons, which then excite the phosphor coating on the front of the display.[128][129]
Xenon is used as a "starter gas" in high pressure sodium lamps. It has the lowest thermal conductivity and lowest ionization potential of all the non-radioactive noble gases. As a noble gas, it does not interfere with the chemical reactions occurring in the operating lamp. The low thermal conductivity minimizes thermal losses in the lamp while in the operating state, and the low ionization potential causes the breakdown voltage of the gas to be relatively low in the cold state, which allows the lamp to be more easily started.[130]
Lasers
In 1962, a group of researchers at Bell Laboratories discovered laser action in xenon,[131] and later found that the laser gain was improved by adding helium to the lasing medium.[132][133] The first excimer laser used a xenon dimer (Xe2) energized by a beam of electrons to produce stimulated emission at an ultraviolet wavelength of 176 nm.[26] Xenon chloride and xenon fluoride have also been used in excimer (or, more accurately, exciplex) lasers.[134] The xenon chloride excimer laser has been employed, for example, in certain dermatological uses.[135]
Medical
Anesthesia
Xenon has been used as a general anesthetic. Although it is expensive, anesthesia machines that can deliver xenon are about to appear on the European market, because advances in recovery and recycling of xenon have made it economically viable.[61][136]
Xenon affects many different receptors and neurotransmitters, like many theoretically multi-modal inhalation anesthetics they are likely complementary. Xenon is a high affinity glycine-site NMDA antagonist. However, xenon distinguishes itself from other clinically used NMDA antagonists in its lack of neurotoxicity and ability to inhibit the neurotoxicity of ketamine and nitrous oxide. Xenon does not provoke the release of dopamine from the nucleus accumbens and inhibits the meso-accumbal DA efflux from ketamine and nitrous oxide. Xenon activates the two-pore domain potassium channel TREK-1. Xenon inhibits nicotinic acetylcholine alpha4beta2 receptors which contribute to spinally mediated analgesia.
Xenon is a competitive inhibitor of serotonin 5HT3. While neither anesthetic nor antinociceptive this activity reduces anesthesia-emergent nausea and vomiting.
Two physiological mechanisms for xenon anesthesia have been proposed. The first one involves the inhibition of the calcium ATPase pump—the mechanism cells use to remove calcium (Ca2+)—in the cell membrane of synapses.[137] This results from a conformational change when xenon binds to nonpolar sites inside the protein.[138]
Xenon has a minimum alveolar concentration (MAC) of 72% at age 40, making it 44% more potent than N2O as an anesthetic.[139] Thus it can be used in concentrations with oxygen that have a lower risk of hypoxia. Unlike nitrous oxide (N2O), xenon is not a greenhouse gas and so it is also viewed as environmentally friendly.[140] Xenon vented into the atmosphere is being returned to its original source, so no environmental impact is likely.
Neuroprotectant
Xenon holds great promise as a rapid-acting, low toxicity, high potency neuroprotectant. Xenon is a high affinity antagonist at the NMDA receptor glycine site.
Xenon is cardioprotective in ischemia-reperfusion conditions by inducing pharmacologic non-ischemic preconditioning. Xenon is cardioprotective by activating PKC-epsilon & downstream p38-MAPK. http://www.ncbi.nlm.nih.gov/pubmed/15644876 Xenon mimics neuronal ischemic preconditioning by activating ATP sensitive potassium channels. http://www.ncbi.nlm.nih.gov/pubmed/19352153 Xenon allosterically reduces ATP mediated channel activation inhibition independently of the sulfonylurea receptor1 subunit, increasing KATP open-channel time and frequency. http://www.ncbi.nlm.nih.gov/pubmed/20179498 Xenon upregulates hypoxia inducible factor 1 alpha (HIF1a). http://www.isrn.com/journals/anesthesiology/2011/510297/
Xenon gas was added as an ingredient of the ventilation mix for a newborn baby at St. Michael's Hospital, Bristol, England, whose life chances were otherwise very compromised, and was successful, leading to the authorisation of clinical trials for similar cases.[141] The treatment is done simultaneously with cooling the body temperature to 33.5 °C.[142]
Imaging
Gamma emission from the radioisotope 133Xe of xenon can be used to image the heart, lungs, and brain, for example, by means of single photon emission computed tomography. 133Xe has also been used to measure blood flow.[143][144][145]
Xenon, particularly hyperpolarized 129Xe, is a useful contrast agent for magnetic resonance imaging (MRI). In the gas phase, it can be used to image empty space such as cavities in a porous sample or alveoli in lungs. Hyperpolarization renders 129Xe much more detectable via magnetic resonance imaging and has been used for studies of the lungs and other tissues. It can be used, for example, to trace the flow of gases within the lungs.[146][147] Because xenon is soluble in water and also in hydrophobic solvents, it can be used to image various soft living tissues.[148][149][150]
NMR spectroscopy
Because of the atom's large, flexible outer electron shell, the NMR spectrum changes in response to surrounding conditions, and can therefore be used as a probe to measure the chemical circumstances around the xenon atom. For instance xenon dissolved in water, xenon dissolved in hydrophobic solvent, and xenon associated with certain proteins can be distinguished by NMR.[151][152]
Hyperpolarized xenon can be used by surface-chemists. Normally, it is difficult to characterize surfaces using NMR, because signals from the surface of a sample will be overwhelmed by signals from the far-more-numerous atomic nuclei in the bulk. However, nuclear spins on solid surfaces can be selectively polarized, by transferrering spin polarization to them from hyperpolarized xenon gas. This makes the surface signals strong enough to measure, and distinguishes them from bulk signals.[153][154]
Other
In nuclear energy applications, xenon is used in bubble chambers,[155] probes, and in other areas where a high molecular weight and inert nature is desirable. A by-product of nuclear weapon testing is the release of radioactive xenon-133 and xenon-135. The detection of these isotopes is used to monitor compliance with nuclear test ban treaties,[156] as well as to confirm nuclear test explosions by states such as North Korea.[157]

Liquid xenon is being used in calorimeters[158] for measurements of gamma rays as well as a medium for detecting hypothetical weakly interacting massive particles, or WIMPs. When a WIMP collides with a xenon nucleus, it should, theoretically, strip an electron and create a primary scintillation. By using xenon, this burst of energy could then be readily distinguished from similar events caused by particles such as cosmic rays.[28] However, the XENON experiment at the Gran Sasso National Laboratory in Italy and the ZEPLIN-II and ZEPLIN-III experiments at the Boulby Underground Laboratory in the UK have thus far failed to find any confirmed WIMPs. Even if no WIMPs are detected, the experiments will serve to constrain the properties of dark matter and some physics models.[159][160] The current detector at the Gran Sasso facility has demonstrated sensitivity comparable to that of the best cryogenic detectors, and the sensitivity was expected to be increased by an order of magnitude in 2009.[161]
Xenon is the preferred propellant for ion propulsion of spacecraft because of its low ionization potential per atomic weight, and its ability to be stored as a liquid at near room temperature (under high pressure) yet be easily converted back into a gas to feed the engine. The inert nature of xenon makes it environmentally friendly and less corrosive to an ion engine than other fuels such as mercury or caesium. Xenon was first used for satellite ion engines during the 1970s.[162] It was later employed as a propellant for JPL's Deep Space 1 probe, Europe's SMART-1 spacecraft[29] and for the three ion propulsion engines on NASA's Dawn Spacecraft.[163]
Chemically, the perxenate compounds are used as oxidizing agents in analytical chemistry. Xenon difluoride is used as an etchant for silicon, particularly in the production of microelectromechanical systems (MEMS).[164] The anticancer drug 5-fluorouracil can be produced by reacting xenon difluoride with uracil.[165] Xenon is also used in protein crystallography.</ref> Applied at pressures from 0.5 to 5 MPa (5 to 50 atm) to a protein crystal, xenon atoms bind in predominantly hydrophobic cavities, often creating a high quality, isomorphous, heavy-atom derivative, which can be used for solving the phase problem.[166][167]
Precautions
Many oxygen-containing xenon compounds are toxic due to their strong oxidative properties, and explosive due to their tendency to break down into elemental xenon plus diatomic oxygen (O2), which contains much stronger chemical bonds than the xenon compounds.[168]
Xenon gas can be safely kept in normal sealed glass or metal containers at standard temperature and pressure. However, it readily dissolves in most plastics and rubber, and will gradually escape from a container sealed with such materials.[169] Xenon is non-toxic, although it does dissolve in blood and belongs to a select group of substances that penetrate the blood–brain barrier, causing mild to full surgical anesthesia when inhaled in high concentrations with oxygen.[168]
At 169 m/s, the speed of sound in xenon gas is slower than that in air[170] due to the slower average speed of the heavy xenon atoms compared to nitrogen and oxygen molecules. Hence, xenon lowers the resonant frequencies of the vocal tract when inhaled. This produces a characteristic lowered voice timbre, an effect opposite to the high-timbred voice caused by inhalation of helium. Like helium, xenon does not satisfy the body's need for oxygen. Xenon is both a simple asphyxiant and an anesthetic more powerful than nitrous oxide; consequently, many universities no longer allow the voice stunt as a general chemistry demonstration. As xenon is expensive, the gas sulfur hexafluoride, which is similar to xenon in molecular weight (146 versus 131), is generally used in this stunt, and is an asphyxiant without being anesthetic.[171]
It is possible to safely breathe heavy gases such as xenon or sulfur hexafluoride when they are in a mixture with oxygen; the oxygen comprising at least 20% of the mixture. Xenon at 80% concentration along with 20% oxygen rapidly produces the unconsciousness of general anesthesia (and has been used for this, as discussed above). Breathing mixes gases of different densities very effectively and rapidly so that heavier gases are purged along with the oxygen, and do not accumulate at the bottom of the lungs.[172] There is, however, a danger associated with any heavy gas in large quantities: it may sit invisibly in a container, and if a person enters a container filled with an odorless, colorless gas, they may find themselves breathing it unknowingly. Xenon is rarely used in large enough quantities for this to be a concern, though the potential for danger exists any time a tank or container of xenon is kept in an unventilated space.[173]
See also
References
- ^ "xenon". Oxford English Dictionary. Vol. 20 (2nd ed.). Oxford University Press. 1989.
- ^ "Xenon". Dictionary.com Unabridged. 2010. Retrieved May 6, 2010.
- ^ "Standard Atomic Weights: Xenon". CIAAW. 1999.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ "Xenon". Gas Encyclopedia. Air Liquide. 2009.
- ^ a b Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, Florida: CRC Press. p. 4.123. ISBN 1-4398-5511-0.
- ^ Hwang, Shuen-Cheng; Weltmer, William R. (2000). "Helium Group Gases". Kirk-Othmer Encyclopedia of Chemical Technology. Wiley. pp. 343–383. doi:10.1002/0471238961.0701190508230114.a01. ISBN 0-471-23896-1.
- ^ Xe(0) has been observed in tetraxenonogold(II) (AuXe42+).
- ^ Harding, Charlie; Johnson, David Arthur; Janes, Rob (2002). Elements of the p block. Great Britain: Royal Society of Chemistry. pp. 93–94. ISBN 0-85404-690-9.
- ^ Magnetic susceptibility of the elements and inorganic compounds, in Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton, Florida: CRC Press. ISBN 0-8493-0486-5.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ "Observation of two-neutrino double electron capture in 124Xe with XENON1T". Nature. 568 (7753): 532–535. 2019. doi:10.1038/s41586-019-1124-4.
- ^ Albert, J. B.; Auger, M.; Auty, D. J.; Barbeau, P. S.; Beauchamp, E.; Beck, D.; Belov, V.; Benitez-Medina, C.; Bonatt, J.; Breidenbach, M.; Brunner, T.; Burenkov, A.; Cao, G. F.; Chambers, C.; Chaves, J.; Cleveland, B.; Cook, S.; Craycraft, A.; Daniels, T.; Danilov, M.; Daugherty, S. J.; Davis, C. G.; Davis, J.; Devoe, R.; Delaquis, S.; Dobi, A.; Dolgolenko, A.; Dolinski, M. J.; Dunford, M.; et al. (2014). "Improved measurement of the 2νββ half-life of 136Xe with the EXO-200 detector". Physical Review C. 89. arXiv:1306.6106. Bibcode:2014PhRvC..89a5502A. doi:10.1103/PhysRevC.89.015502.
- ^ Redshaw, M.; Wingfield, E.; McDaniel, J.; Myers, E. (2007). "Mass and Double-Beta-Decay Q Value of 136Xe". Physical Review Letters. 98 (5): 53003. Bibcode:2007PhRvL..98e3003R. doi:10.1103/PhysRevLett.98.053003.
- ^ Staff (2007). "Xenon". Columbia Electronic Encyclopedia (6th ed.). Columbia University Press. Retrieved 2007-10-23.
- ^ a b Husted, Robert; Boorman, Mollie (December 15, 2003). "Xenon". Los Alamos National Laboratory, Chemical Division. Retrieved 2007-09-26.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Rabinovich, Viktor Abramovich (1988). Thermophysical properties of neon, argon, krypton, and xenon (English-language ed.). Washington, DC: Hemisphere Publishing Corp. ISBN 0-89116-675-0. Retrieved 2009-04-02.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)—National Standard Reference Data Service of the USSR. Volume 10. - ^ a b Freemantel, Michael (August 25, 2003). "Chemistry at its Most Beautiful" (PDF). Chemical & Engineering News. Retrieved 2007-09-13.
- ^ a b Kaneoka, Ichiro (1998). "Xenon's Inside Story". Science. 280 (5365): 851–852. doi:10.1126/science.280.5365.851b.
- ^ a b Stacey, Weston M. (2007). Nuclear Reactor Physics. Wiley-VCH. p. 213. ISBN 3-527-40679-4.
- ^ a b c Burke, James (2003). Twin Tracks: The Unexpected Origins of the Modern World. Oxford University Press. p. 33. ISBN 0-7432-2619-4.
- ^ a b Mellor, David (2000). Sound Person's Guide to Video. Focal Press. p. 186. ISBN 0-240-51595-1.
- ^ Sanders, Robert D.; Ma, Daqing; Maze, Mervyn (2005). "Xenon: elemental anaesthesia in clinical practice". British Medical Bulletin. 71 (1): 115–35. doi:10.1093/bmb/ldh034. PMID 15728132.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Basov, N. G. (1971). "Stimulated Emission in the Vacuum Ultraviolet Region". Soviet Journal of Quantum Electronics. 1 (1): 18–22. Bibcode:1971QuEle...1...18B. doi:10.1070/QE1971v001n01ABEH003011.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Toyserkani, E. (2004). Laser Cladding. CRC Press. p. 48. ISBN 0-8493-2172-7.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Ball, Philip (May 1, 2002). "Xenon outs WIMPs". Nature. Retrieved 2007-10-08.
- ^ a b Saccoccia, G. (August 31, 2006). "Ion engine gets SMART-1 to the Moon". ESA. Retrieved 2007-10-01.
{{cite news}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ramsay, W.; Travers, M. W. (1898). "On the extraction from air of the companions of argon, and neon". Report of the Meeting of the British Association for the Advancement of Science: 828.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gagnon, Steve. "It's Elemental – Xenon". Thomas Jefferson National Accelerator Facility. Retrieved 2007-06-16.
- ^ Anonymous (1904). Daniel Coit Gilman, Harry Thurston Peck, Frank Moore Colby (ed.). The New International Encyclopædia. Dodd, Mead and Company. p. 906.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ Staff (1991). The Merriam-Webster New Book of Word Histories. Merriam-Webster, Inc. p. 513. ISBN 0-87779-603-3.
- ^ Ramsay, William (1902). "An Attempt to Estimate the Relative Amounts of Krypton and of Xenon in Atmospheric Air". Proceedings of the Royal Society of London. 71 (467–476): 421–426. doi:10.1098/rspl.1902.0121.
- ^ Anonymous. "History". Millisecond Cinematography. Archived from the original on 2006-08-22. Retrieved 2007-11-07.
- ^ Paschotta, Rüdiger (November 1, 2007). "Lamp-pumped lasers". Encyclopedia of Laser Physics and Technology. RP Photonics. Retrieved 2007-11-07.
- ^ Marx, Thomas; Schmidt, Michael; Schirmer, Uwe; Reinelt, Helmut (2000). "Xenon anesthesia" (PDF). Journal of the Royal Society of Medicine. 93 (10): 513–7. PMC 1298124. PMID 11064688. Retrieved 2007-10-02.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bartlett, Neil; Lohmann, D. H. (1962). "Dioxygenyl hexafluoroplatinate (V), O2+[PtF6]–". Proceedings of the Chemical Society (3). London: Chemical Society: 115. doi:10.1039/PS9620000097.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bartlett, N. (1962). "Xenon hexafluoroplatinate (V) Xe+[PtF6]–". Proceedings of the Chemical Society (6). London: Chemical Society: 218. doi:10.1039/PS9620000197.
- ^ Graham, L. (2000). "Concerning the nature of XePtF6". Coordination Chemistry Reviews. 197 (1): 321–334. doi:10.1016/S0010-8545(99)00190-3.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Holleman, A. F. (2001). Inorganic Chemistry. translated by Mary Eagleson and William Brewer. San Diego: Academic Press. ISBN 0-12-352651-5.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|editors=ignored (|editor=suggested) (help); translation of Lehrbuch der Anorganischen Chemie, originally founded by A. F. Holleman, continued by Egon Wiberg, edited by Nils Wiberg, Berlin: de Gruyter, 1995, 34th edition, ISBN 3-11-012641-9. - ^ Steel, Joanna (2007). "Biography of Neil Bartlett". College of Chemistry, University of California, Berkeley. Retrieved 2007-10-25.
- ^ Bartlett, Neil (2003-09-09). "The Noble Gases". Chemical & Engineering News. 81 (36). American Chemical Society. Retrieved 2007-10-01.
- ^ Khriachtchev, Leonid (2000-08-24). "A stable argon compound". Nature. 406 (6798): 874–6. doi:10.1038/35022551. PMID 10972285. Retrieved 2008-06-04.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Lynch, C. T.; Summitt, R.; Sliker, A. (1980). CRC Handbook of Materials Science. CRC Press. ISBN 0-87819-231-X.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ MacKenzie, D. R. (1963). "Krypton Difluoride: Preparation and Handling". Science. 141 (3586): 1171. Bibcode:1963Sci...141.1171M. doi:10.1126/science.141.3586.1171. PMID 17751791.
- ^ Paul R. Fields, Lawrence Stein, and Moshe H. Zirin (1962). "Radon Fluoride". Journal of the American Chemical Society. 84 (21): 4164–4165. doi:10.1021/ja00880a048.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Xenon". Periodic Table Online. CRC Press. Archived from the original on April 10, 2007. Retrieved 2007-10-08.
- ^ Moody, G. J. (1974). "A Decade of Xenon Chemistry". Journal of Chemical Education. 51 (10): 628–630. Bibcode:1974JChEd..51..628M. doi:10.1021/ed051p628. Retrieved 2007-10-16.
- ^ Williams, David R. (April 19, 2007). "Earth Fact Sheet". NASA. Retrieved 2007-10-04.
- ^ a b Aprile, Elena (2006). Noble Gas Detectors. Wiley-VCH. pp. 8–9. ISBN 3-527-60963-6.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Caldwell, W. A. (1997). "Structure, bonding and geochemistry of xenon at high pressures". Science. 277 (5328): 930–933. doi:10.1126/science.277.5328.930.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Fontes, E. "Golden Anniversary for Founder of High-pressure Program at CHESS". Cornell University. Retrieved 2009-05-30.
- ^ Eremets, Mikhail I.; Gregoryanz, Eugene A.; Struzhkin, Victor V.; Mao, Ho-Kwang; Hemley, Russell J.; Mulders, Norbert; Zimmerman, Neil M. (2000). "Electrical Conductivity of Xenon at Megabar Pressures". Physical Review Letters. 85 (13): 2797–800. Bibcode:2000PhRvL..85.2797E. doi:10.1103/PhysRevLett.85.2797. PMID 10991236.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bader, Richard F. W. "An Introduction to the Electronic Structure of Atoms and Molecules". McMaster University. Retrieved 2007-09-27.
- ^ Talbot, John. "Spectra of Gas Discharges". Rheinisch-Westfälische Technische Hochschule Aachen. Retrieved 2006-08-10.
- ^ Watts, William Marshall (1904). An Introduction to the Study of Spectrum Analysis. London: Longmans, Green, and co.
- ^ Hwang, Shuen-Cheng (2005). "Noble Gases". Kirk-Othmer Encyclopedia of Chemical Technology (5th ed.). Wiley. doi:10.1002/0471238961.0701190508230114.a01. ISBN 0-471-48511-X.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Kerry, Frank G. (2007). Industrial Gas Handbook: Gas Separation and Purification. CRC Press. pp. 101–103. ISBN 0-8493-9005-2.
- ^ "Xenon – Xe". CFC StarTec LLC. August 10, 1998. Retrieved 2007-09-07.
- ^ a b Singh, Sanjay (May 15, 2005). "Xenon: A modern anaesthetic". Indian Express Newspapers Limited. Archived from the original on 2007-08-13. Retrieved 2007-10-10.
- ^ a b Häussinger, Peter (2001). "Noble Gases". Ullmann's Encyclopedia of Industrial Chemistry (6th ed.). Wiley. doi:10.1002/14356007.a17_485. ISBN 3-527-20165-3.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Arnett, David (1996). Supernovae and Nucleosynthesis. Princeton, New Jersey: Princeton University Press. ISBN 0-691-01147-8.
- ^ Mahaffy, P. R. (2000). "Noble gas abundance and isotope ratios in the atmosphere of Jupiter from the Galileo Probe Mass Spectrometer". Journal of Geophysical Research. 105 (E6): 15061–15072. Bibcode:2000JGR...10515061M. doi:10.1029/1999JE001224.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Owen, Tobias (1999). "A low-temperature origin for the planetesimals that formed Jupiter". Nature. 402 (6759): 269–70. Bibcode:1999Natur.402..269O. doi:10.1038/46232. PMID 10580497.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Sanloup, Chrystèle; et al. (2005). "Retention of Xenon in Quartz and Earth's Missing Xenon". Science. 310 (5751): 1174–7. Bibcode:2005Sci...310.1174S. doi:10.1126/science.1119070. PMID 16293758.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Clayton, Donald D. (1983). Principles of Stellar Evolution and Nucleosynthesis. University of Chicago Press. ISBN 0-226-10953-4.
- ^ Heymann, D. (March 19–23, 1979). "Xenon from intermediate zones of supernovae". Proceedings 10th Lunar and Planetary Science Conference. Houston, Texas: Pergamon Press, Inc. pp. 1943–1959. Bibcode:1979LPSC...10.1943H.
{{cite conference}}: Unknown parameter|booktitle=ignored (|book-title=suggested) (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Beer, H.; Kaeppeler, F.; Reffo, G.; Venturini, G. (1983). "Neutron capture cross-sections of stable xenon isotopes and their application in stellar nucleosynthesis". Astrophysics and Space Science. 97 (1): 95–119. Bibcode:1983Ap&SS..97...95B. doi:10.1007/BF00684613.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Pignatari, M.; Gallino; Straniero; Davis (2004). "The origin of xenon trapped in presolar mainstream SiC grains". Memorie della Societa Astronomica Italiana. 75: 729–734. Bibcode:2004MmSAI..75..729P.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Caldwell, Eric (2004). "Periodic Table – Xenon". Resources on Isotopes. USGS. Retrieved 2007-10-08.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ Rajam, J. B. (1960). Atomic Physics (7th ed.). Delhi: S. Chand and Co. ISBN 81-219-1809-X.
- ^ Barabash, A. S. (2002). "Average (Recommended) Half-Life Values for Two-Neutrino Double-Beta Decay". Czechoslovak Journal of Physics. 52 (4): 567–573. arXiv:nucl-ex/0203001. Bibcode:2002CzJPh..52..567B. doi:10.1023/A:1015369612904.
- ^ Cite error: The named reference
EXOwas invoked but never defined (see the help page). - ^ Otten, Ernst W. (2004). "Take a breath of polarized noble gas". Europhysics News. 35 (1): 16. Bibcode:2004ENews..35...16O. doi:10.1051/epn:2004109.
- ^ Ruset, I. C. (2006). "Optical Pumping System Design for Large Production of Hyperpolarized 129Xe". Physical Review Letters. 96 (5): 053002. Bibcode:2006PhRvL..96e3002R. doi:10.1103/PhysRevLett.96.053002.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Wolber, J. (2000). "On the oxygenation-dependent 129Xe T1 in blood". NMR in Biomedicine. 13 (4): 234–7. doi:10.1002/1099-1492(200006)13:4<234::AID-NBM632>3.0.CO;2-K. PMID 10867702.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Chann, B. (2002). "129Xe-Xe molecular spin relaxation". Physical Review Letters. 88 (11): 113–201. Bibcode:2002PhRvL..88k3201C. doi:10.1103/PhysRevLett.88.113201.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ von Schulthess, Gustav Konrad (1998). The Encyclopaedia of Medical Imaging. Taylor & Francis. p. 194. ISBN 1-901865-13-4.
{{cite encyclopedia}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Warren, W. W. (1966). "Nuclear Quadrupole Relaxation and Chemical Shift of Xe131 in Liquid and Solid Xenon". Physical Review. 148 (1): 402–412. Bibcode:1966PhRv..148..402W. doi:10.1103/PhysRev.148.402.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Staff. "Hanford Becomes Operational". The Manhattan Project: An Interactive History. U.S. Department of Energy. Archived from the original on 2009-12-10. Retrieved 2007-10-10.
- ^ Pfeffer, Jeremy I. (2000). Modern Physics: An Introductory Text. Imperial College Press. pp. 421 ff. ISBN 1-86094-250-4.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Laws, Edwards A. (2000). Aquatic Pollution: An Introductory Text. John Wiley and Sons. p. 505. ISBN 0-471-34875-9.
- ^ Staff (April 9, 1979). "A Nuclear Nightmare". Time. Retrieved 2007-10-09.
- ^ Clayton, Donald D. (1983). Principles of Stellar Evolution and Nucleosynthesis (2nd ed.). University of Chicago Press. p. 75. ISBN 0-226-10953-4.
- ^ Bolt, B. A.; Packard, R. E.; Price, P. B. (2007). "John H. Reynolds, Physics: Berkeley". The University of California, Berkeley. Retrieved 2007-10-01.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Williams, David R. (September 1, 2004). "Mars Fact Sheet". NASA. Retrieved 2007-10-10.
- ^ Schilling, James. "Why is the Martian atmosphere so thin and mainly carbon dioxide?". Mars Global Circulation Model Group. Retrieved 2007-10-10.
- ^ Zahnle, Kevin J. (1993). "Xenological constraints on the impact erosion of the early Martian atmosphere". Journal of Geophysical Research. 98 (E6): 10, 899–10, 913. Bibcode:1993JGR....9810899Z. doi:10.1029/92JE02941.
- ^ Boulos, M. S. (1971). "The xenon record of extinct radioactivities in the Earth". Science. 174 (4016): 1334–6. Bibcode:1971Sci...174.1334B. doi:10.1126/science.174.4016.1334. PMID 17801897.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Harding, Charlie; Johnson, David Arthur; Janes, Rob (2002). Elements of the p block. Great Britain: Royal Society of Chemistry. pp. 93–94. ISBN 0-85404-690-9.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Dean H Liskow, Henry F I I I Schaefer, Paul S Bagus, Bowen Liu (1973). "Probable nonexistence of xenon monofluoride as a chemically bound species in the gas phase". J Amer Chem Soc. 95 (12): 4056–4057.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Weeks, James L.; Chernick, Cedric; Matheson, Max S. (1962). "Photochemical Preparation of Xenon Difluoride". Journal of the American Chemical Society. 84 (23): 4612. doi:10.1021/ja00882a063.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Streng, L. V.; Streng, A. G. (1965). "Formation of Xenon Difluoride from Xenon and Oxygen Difluoride or Fluorine in Pyrex Glass at Room Temperature". Inorganic Chemistry. 4 (9): 1370–1371. doi:10.1021/ic50031a035.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Tramšek, Melita; Žemva, Boris (December 5, 2006). "Synthesis, Properties and Chemistry of Xenon(II) Fluoride" (PDF). Acta Chimica Slovenica. 53 (2): 105–116. doi:10.1002/chin.200721209. Retrieved 2009-07-18.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ogrin, Tomaz; Bohinc, Matej; Silvnik, Joze (1973). "Melting-point determinations of xenon difluoride-xenon tetrafluoride mixtures". Journal of Chemical and Engineering Data. 18 (4): 402. doi:10.1021/je60059a014.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Scott, Thomas; Eagleson, Mary (1994). "Xenon Compounds". Concise encyclopedia chemistry. Walter de Gruyter. p. 1183. ISBN 3-11-011451-8.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link) - ^ Proserpio, Davide M.; Hoffmann, Roald; Janda, Kenneth C. (1991). "The xenon-chlorine conundrum: van der Waals complex or linear molecule?". Journal of the American Chemical Society. 113 (19): 7184. doi:10.1021/ja00019a014.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Richardson, Nancy A.; Hall, Michael B. (1993). "The potential energy surface of xenon dichloride". The Journal of Physical Chemistry. 97 (42): 10952. doi:10.1021/j100144a009.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brock, D.S..; Schrobilgen, G.J. (2011). "Synthesis of the missing oxide of xenon, XeO2, and its implications for earth's missing xenon". Journal of the American Chemical Society. 133 (16): 110222081739042. doi:10.1021/ja110618g. PMID 21341650.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/471138d, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/471138dinstead. - ^ Zhou, M.; Zhao, Y.; Gong, Y.; Li, J. (2006). "Formation and Characterization of the XeOO+ Cation in Solid Argon". Journal of the American Chemical Society. 128 (8): 2504–5. doi:10.1021/ja055650n. PMID 16492012.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Holloway, John H. (1998). A. G. Sykes (ed.). Advances in Inorganic Chemistry Press. Academic. p. 65. ISBN 0-12-023646-X.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Henderson, W. (2000). Main group chemistry. Great Britain: Royal Society of Chemistry. pp. 152–153. ISBN 0-85404-617-8.
- ^ a b c d Mackay, Kenneth Malcolm; Mackay, Rosemary Ann; Henderson, W. (2002). Introduction to modern inorganic chemistry (6th ed.). CRC Press. pp. 497–501. ISBN 0-7487-6420-8.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Smith, D. F. (1963). "Xenon Oxyfluoride". Science. 140 (3569): 899. Bibcode:1963Sci...140..899S. doi:10.1126/science.140.3569.899. PMID 17810680.
{{cite journal}}: More than one of|pages=and|page=specified (help) - ^ K. O. Christe, D. A. Dixon, J. C. P. Sanders, G. J. Schrobilgen, S. S. Tsai, W. W. Wilson (1995). "On the Structure of the [XeOF5]− Anion and of Heptacoordinated Complex Fluorides Containing One or Two Highly Repulsive Ligands or Sterically Active Free Valence Electron Pairs". Inorg. Chem. 34 (7): 1868–1874. doi:10.1021/ic00111a039.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ K. O. Christe, C. J. Schack, D. Pilipovich (1972). "Chlorine trifluoride oxide. V. Complex formation with Lewis acids and bases". Inorg. Chem. 11 (9): 2205–2208. doi:10.1021/ic50115a044.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Holloway, John H.; Hope, Eric G. (1998). Advances in Inorganic Chemistry. Contributor A. G. Sykes. Academic Press. pp. 61–90. ISBN 0-12-023646-X.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Frohn, H (2004). "C6F5XeF, a versatile starting material in xenon–carbon chemistry". Journal of Fluorine Chemistry. 125 (6): 981. doi:10.1016/j.jfluchem.2004.01.019.
- ^ Goetschel, Charles T. (1972). "Reaction of xenon with dioxygenyl tetrafluoroborate. Preparation of FXe-BF2". Journal of the American Chemical Society. 94 (9): 3018. doi:10.1021/ja00764a022.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Li, Wai-Kee; Zhou, Gong-Du; Mak, Thomas C. W. (2008). Advanced Structural Inorganic Chemistry. Oxford University Press. p. 678. ISBN 0-19-921694-0.
{{cite book}}: Unknown parameter|editors=ignored (|editor=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Gerber, R. B. (2004). "Formation of novel rare-gas molecules in low-temperature matrices". Annual Review of Physical Chemistry. 55 (1): 55–78. Bibcode:2004ARPC...55...55G. doi:10.1146/annurev.physchem.55.091602.094420. PMID 15117247.
- ^ Khriachtchev, Leonid (2008). "A Small Neutral Molecule with Two Noble-Gas Atoms: HXeOXeH". Journal of the American Chemical Society. 130 (19): 6114–8. doi:10.1021/ja077835v. PMID 18407641.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Pettersson, Mika (1999). "A Chemical Compound Formed from Water and Xenon: HXeOH". Journal of the American Chemical Society. 121 (50): 11904–11905. doi:10.1021/ja9932784.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Pauling, L. (1961). "A molecular theory of general anesthesia". Science. 134 (3471): 15–21. Bibcode:1961Sci...134...15P. doi:10.1126/science.134.3471.15. PMID 13733483. Reprinted as Pauling, Linus; Kamb, Barclay, ed. (2001). Linus Pauling: Selected Scientific Papers. Vol. 2. River Edge, New Jersey: World Scientific. pp. 1328–1334. ISBN 981-02-2940-2.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ Henderson, W. (2000). Main group chemistry. Great Britain: Royal Society of Chemistry. p. 148. ISBN 0-85404-617-8.
- ^ Ikeda, Tomoko (November 23, 2000). "Distortion of Host Lattice in Clathrate Hydrate as a Function of Guest Molecule and Temperature". Journal of Physical Chemistry A. 104 (46): 10623–10630. doi:10.1021/jp001313j.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ McKay, C. P. (2003). "Clathrate formation and the fate of noble and biologically useful gases in Lake Vostok, Antarctica". Geophysical Letters. 30 (13): 35. Bibcode:2003GeoRL..30m..35M. doi:10.1029/2003GL017490.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Barrer, R. M. (1957). "Non-Stoichiometric Clathrate of Water". Proceedings of the Royal Society of London. 243 (1233): 172–189. Bibcode:1957RSPSA.243..172B. doi:10.1098/rspa.1957.0213.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Frunzi, Michael (2007). "Effect of Xenon on Fullerene Reactions". Journal of the American Chemical Society. 129 (43): 13343. doi:10.1021/ja075568n. PMID 17924634.
{{cite journal}}: More than one of|pages=and|page=specified (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Silfvast, William Thomas (2004). Laser Fundamentals. Cambridge University Press. ISBN 0-521-83345-0.
- ^ Webster, John G. (1998). The Measurement, Instrumentation, and Sensors Handbook. Springer. ISBN 3-540-64830-5.
- ^ McGhee, Charles (1997). Excimer Lasers in Ophthalmology. Informa Health Care. ISBN 1-85317-253-7.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Staff (2007). "Xenon Applications". Praxair Technology. Retrieved 2007-10-04.
- ^ Baltás, E. (2003). "A xenon-iodine electric discharge bactericidal lamp". Technical Physics Letters. 29 (10): 871–872. Bibcode:2003TePhL..29..871S. doi:10.1134/1.1623874.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Skeldon, M.D. (1997). "Thermal distortions in laser-diode- and flash-lamp-pumped Nd:YLF laser rods" (PDF). LLE Review. 71: 137–144. Archived from the original (PDF) on October 16, 2003. Retrieved 2007-02-04.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Anonymous. "The plasma behind the plasma TV screen". Plasma TV Science. Retrieved 2007-10-14.
- ^ Marin, Rick (March 21, 2001). "Plasma TV: That New Object Of Desire". The New York Times. Retrieved 2009-04-03.
- ^ Waymouth, John (1971). Electric Discharge Lamps. Cambridge, MA: The M.I.T. Press. ISBN 0-262-23048-8.
- ^ Patel, C. K. N. (August 1, 1962). "Infrared spectroscopy using stimulated emission techniques". Physical Review Letters. 9 (3): 102–104. Bibcode:1962PhRvL...9..102P. doi:10.1103/PhysRevLett.9.102.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Patel, C. K. N. (December 1, 1962). "High gain gaseous (Xe-He) optical masers". Applied Physics Letters. 1 (4): 84–85. Bibcode:1962ApPhL...1...84P. doi:10.1063/1.1753707.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Bennett, Jr., W. R. (1962). "Gaseous optical masers". Applied Optics Supplement. 1: 24–61.
- ^ "Laser Output". University of Waterloo. Retrieved 2007-10-07.
- ^ Baltás, E. (2006). "Treatment of atopic dermatitis with the xenon chloride excimer laser". Journal of the European Academy of Dermatology and Venereology. 20 (6): 657–60. doi:10.1111/j.1468-3083.2006.01495.x. PMID 16836491.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Tonner, P. H. (2006). "Xenon: one small step for anaesthesia ... ? (editorial review)". Current Opinion in Anaesthesiology. 19 (4): 382–4. doi:10.1097/01.aco.0000236136.85356.13. PMID 16829718.
- ^ Franks, JJ (1995). "Halothane, isoflurane, xenon, and nitrous oxide inhibit calcium ATPase pump activity in rat brain synaptic plasma membranes". Anesthesiology. 82 (1): 108–17. doi:10.1097/00000542-199501000-00015. PMID 7832292. Retrieved 2010-09-15.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Lopez, MM (1995). "How do volatile anesthetics inhibit Ca2+-ATPases?". Journal of Biological Chemistry. 270 (47): 28239–45. doi:10.1074/jbc.270.47.28239. PMID 7499320. Retrieved 2010-09-15.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Nickalls, R. W. D. "Age‐related iso‐MAC charts for isoflurane, sevoflurane and desflurane in man". British Journal of Anesthesiology.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Goto, T (2003). "Will xenon be a stranger or a friend?: the cost, benefit, and future of xenon anesthesia". Anesthesiology. 98 (1): 1–2. doi:10.1097/00000542-200301000-00002. PMID 12502969. Retrieved 2010-09-15.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Staff (April 9, 2010). "First baby given xenon gas to prevent brain injury". BBC News. Retrieved 2010-04-09.
- ^ Newman, Sian. "Xenon gas used in a bid to reduce brain injury in newborns". Swansea University. Retrieved 2011-10-19.
- ^ Van Der Wall, Ernst (1992). What's New in Cardiac Imaging?: SPECT, PET, and MRI. Springer. ISBN 0-7923-1615-0.
- ^ Frank, John (1999). "Introduction to imaging: The chest". Student BMJ. 12: 1–44. Retrieved 2008-06-04.
- ^ Chandak, Puneet K. (July 20, 1995). "Brain SPECT: Xenon-133". Brigham RAD. Retrieved 2008-06-04.
- ^ Albert, M. S. (1998). "Development of hyperpolarized noble gas MRI". Nuclear Instruments and Methods in Physics Research A. 402 (2–3): 441–53. Bibcode:1998NIMPA.402..441A. doi:10.1016/S0168-9002(97)00888-7. PMID 11543065.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Irion, Robert (March 23, 1999). "Head Full of Xenon?". Science News. Archived from the original on January 17, 2004. Retrieved 2007-10-08.
- ^ Wolber, J.; Rowland, I. J.; Leach, M. O.; Bifone, A. (1998). "Intravascular delivery of hyperpolarized 129Xenon for in vivo MRI". Applied Magnetic Resonance. 15 (3–4): 343–352. doi:10.1007/BF03162020.
- ^ Driehuys, B.; Möller, H.E.; Cleveland, Z.I.; Pollaro, J.; Hedlund, L.W.; (2009). "Pulmonary perfusion and xenon gas exchange in rats: MR imaging with intravenous injection of hyperpolarized 129Xe". Radiology. 252 (2): 386–93. doi:10.1148/radiol.2522081550. PMC 2753782. PMID 19703880. SSRN 2.
{{cite journal}}: Check|ssrn=value (help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Cleveland, Z.I.; Möller, H.E.; Hedlund, L.W.; Driehuys, B. (2009). "Continuously infusing hyperpolarized 129Xe into flowing aqueous solutions using hydrophobic gas exchange membranes". The journal of physical chemistry. 113 (37): 12489–99. doi:10.1021/jp9049582. PMC 2747043. PMID 19702286.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Luhmer, M.; Dejaegere, A.; Reisse, J. (1989). "Interpretation of the solvent effect on the screening constant of Xe-129". Magnetic Resonance in Chemistry. 27 (10): 950. doi:10.1002/mrc.1260271009.
- ^ Rubin, Seth M.; Spence, Megan M.; Goodson, Boyd M.; Wemmer, David E.; Pines, Alexander (August 15, 2000). "Evidence of nonspecific surface interactions between laser-polarized xenon and myoglobin in solution". Proceedings of the National Academy of Science USA. 97 (17): 9472–5. Bibcode:2000PNAS...97.9472R. doi:10.1073/pnas.170278897. PMC 16888. PMID 10931956.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Raftery, Daniel; MacNamara, Ernesto; Fisher, Gregory; Rice, Charles V.; Smith, Jay (1997). "Optical Pumping and Magic Angle Spinning: Sensitivity and Resolution Enhancement for Surface NMR Obtained with Laser-Polarized Xenon". Journal of the American Chemical Society. 119 (37): 8746. doi:10.1021/ja972035d.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gaede, H. C.; Song, Y. -Q.; Taylor, R. E.; Munson, E. J.; Reimer, J. A.; Pines, A. (1995). "High-field cross polarization NMR from laser-polarized xenon to surface nuclei". Applied Magnetic Resonance. 8 (3–4): 373. doi:10.1007/BF03162652.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Galison, Peter Louis (1997). Image and Logic: A Material Culture of Microphysics. University of Chicago Press. p. 339. ISBN 0-226-27917-0.
- ^ Fontaine, J.-P.; Pointurier, F.; Blanchard, X.; Taffary, T. (2004). "Atmospheric xenon radioactive isotope monitoring". Journal of Environmental Radioactivity. 72 (1–2): 129–35. doi:10.1016/S0265-931X(03)00194-2. PMID 15162864.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Garwin, Richard L.; von Hippel Frank N. (2006). "A Technical Analysis: Deconstructing North Korea's October 9 Nuclear Test". Arms Control Today. 38 (9). Arms Control Association. Retrieved 2009-03-26.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Gallucci, G. (2009). "The MEG liquid xenon calorimeter". Journal of Physics: Conference Series. 160 (1): 012011. Bibcode:2009JPhCS.160a2011G. doi:10.1088/1742-6596/160/1/012011.
- ^ Schumann, Marc (October 10, 2007). "XENON announced new best limits on Dark Matter". Rice University. Retrieved 2007-10-08.
- ^ Lebedenko, V. N.; et al. (2009). "Results from the first science run of the ZEPLIN-III dark matter search experiment". Physical Review D. 80 (5): 052010. Bibcode:2009PhRvD..80e2010L. doi:10.1103/PhysRevD.80.052010.
- ^ Boyd, Jade (August 23, 2007). "Rice physicists go deep for 'dark matter'". Hubble News Desk. Retrieved 2007-10-08.
- ^ Zona, Kathleen (March 17, 2006). "Innovative Engines: Glenn Ion Propulsion Research Tames the Challenges of 21st century Space Travel". NASA. Retrieved 2007-10-04.
- ^ "Dawn Launch: Mission to Vesta and Ceres" (PDF). NASA. Retrieved 2007-10-01.
- ^ Brazzle, J. D. (July 28 – August 1, 1975). "Modeling and Characterization of Sacrificial Polysilicon Etching Using Vapor-Phase Xenon Difluoride". Proceedings 17th IEEE International Conference on Micro Electro Mechanical Systems (MEMS). Maastricht, Netherlands: IEEE. pp. 737–740. ISBN 978-0-7803-8265-7.
{{cite conference}}: Check date values in:|date=(help); Unknown parameter|booktitle=ignored (|book-title=suggested) (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Staff (2007). "Neil Bartlett and the Reactive Noble Gases". American Chemical Society. Retrieved June 5, 2012.
- ^ Staff (December 21, 2004). "Protein Crystallography: Xenon and Krypton Derivatives for Phasing". Daresbury Laboratory, PX. Archived from the original on 2005-03-16. Retrieved 2007-10-01.
- ^ Drenth, Jan (2007). "The Solution of the Phase Problem by the Isomorphous Replacement Method". Principles of Protein X-Ray Crystallography (3rd ed.). New York: Springer. pp. 123–171. doi:10.1007/0-387-33746-6_7. ISBN 978-0-387-33334-2.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Finkel, A. J. (April 1, 1968). "Metabolic and toxicological effects of water-soluble xenon compounds are studied". NASA. Retrieved 2007-10-04.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ LeBlanc, Adrian D. (1971). "The handling of xenon-133 in clinical studies". Physics in Medicine and Biology. 16 (1): 105–9. Bibcode:1971PMB....16..105L. doi:10.1088/0031-9155/16/1/310. PMID 5579743.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ 169.44 m/s in xenon (at 0°C and 107 KPa), compared to 344 m/s in air. See: Vacek, V. (2001). "Velocity of sound measurements in gaseous per-fluorocarbons and their mixtures". Fluid Phase Equilibria. 185 (1–2): 305–314. doi:10.1016/S0378-3812(01)00479-4.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Spangler, Steve (2007). "Anti-Helium – Sulfur Hexafluoride". Steve Spangler Science. Retrieved 2007-10-04.
- ^ Yamaguchi, K. (2001). "Inhaling Gas With Different CT Densities Allows Detection of Abnormalities in the Lung Periphery of Patients With Smoking-Induced COPD". Chest Journal. 51 (6): 1907–16. doi:10.1378/chest.120.6.1907. PMID 11742921.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Staff (August 1, 2007). "Cryogenic and Oxygen Deficiency Hazard Safety". Stanford Linear Accelerator Center. Archived from the original on June 9, 2007. Retrieved 2007-10-10.
External links
- WebElements.com – Xenon
- USGS Periodic Table – Xenon
- EnvironmentalChemistry.com – Xenon
- Xenon as an anesthetic
- Sir William Ramsay's Nobel-Prize lecture (1904)
Template:Featured article is only for Wikipedia:Featured articles.



