User:Simoncaulton/ANA: Difference between revisions
Simoncaulton (talk | contribs) writing page |
Simoncaulton (talk | contribs) writing page |
||
| Line 28: | Line 28: | ||
[[File:ANA - dsDNA antibody.png|thumb|dsDNA antibody. The variable regions (yellow) are complementary to the dsDNA strands. These antibodies are found commonly in the sera of SLE patients.]] |
[[File:ANA - dsDNA antibody.png|thumb|dsDNA antibody. The variable regions (yellow) are complementary to the dsDNA strands. These antibodies are found commonly in the sera of SLE patients.]] |
||
[[Anti-dsDNA antibodies|Anti-double stranded DNA antibodies]] (anti-dsDNA) are highly associated with SLE. They are a very [[sensitivity and specificity|specific]] marker for the disease, with some studies quoting nearly 100%. Data on [[sensitivity and specificity|sensitivity]] ranges from 25-85%. Anti-dsDNA antibody levels, knows as titres, correlate with disease activity in SLE; high levels indicate more active lupus. The presence of anti-dsDNA antibodies is also associated with [[lupus nephritis]] and evidence suggests that they may even cause this symptom. They may produce large immune complexes with DNA that bind to the [[glomerular basement membrane]] (GBM) or bind directly to antigens in the GBM. Both of these could initiate inflammation within the kidney. The antigen of anti-dsDNA antibodies is [[dsDNA|double stranded DNA]]<ref>{{cite journal|last=Mok|first=CC|coauthors=Lau, CS|title=Pathogenesis of systemic lupus erythematosus.|journal=Journal of clinical pathology|date=2003 Jul|volume=56|issue=7|pages=481-90|pmid=12835292}}</ref><ref name ="pmid10629135"/><ref>{{cite journal|last=Yung|first=S|coauthors=Chan, TM|title=Anti-DNA antibodies in the pathogenesis of lupus nephritis--the emerging mechanisms.|journal=Autoimmunity reviews|date=2008 Feb|volume=7|issue=4|pages=317-21|pmid=18295737}}</ref> |
[[Anti-dsDNA antibodies|Anti-double stranded DNA antibodies]] (anti-dsDNA) are highly associated with SLE. They are a very [[sensitivity and specificity|specific]] marker for the disease, with some studies quoting nearly 100%. Data on [[sensitivity and specificity|sensitivity]] ranges from 25-85%. Anti-dsDNA antibody levels, knows as titres, correlate with disease activity in SLE; high levels indicate more active lupus. The presence of anti-dsDNA antibodies is also associated with [[lupus nephritis]] and evidence suggests that they may even cause this symptom. They may produce large immune complexes with DNA that bind to the [[glomerular basement membrane]] (GBM) or bind directly to antigens in the GBM. Both of these could initiate inflammation within the kidney, and also may [[complement fixation|fix complement]]. The antigen of anti-dsDNA antibodies is [[dsDNA|double stranded DNA]]<ref>{{cite journal|last=Mok|first=CC|coauthors=Lau, CS|title=Pathogenesis of systemic lupus erythematosus.|journal=Journal of clinical pathology|date=2003 Jul|volume=56|issue=7|pages=481-90|pmid=12835292}}</ref><ref name ="pmid10629135"/><ref>{{cite journal|last=Yung|first=S|coauthors=Chan, TM|title=Anti-DNA antibodies in the pathogenesis of lupus nephritis--the emerging mechanisms.|journal=Autoimmunity reviews|date=2008 Feb|volume=7|issue=4|pages=317-21|pmid=18295737}}</ref> |
||
| ⚫ | |||
===Anti-histone antibodies=== |
|||
Anti-histone antibodies are found in the serum of up to 75-95% of [[drug induced lupus]] patients and 75% of idiopathic SLE. Unlike anti-dsDNA antibodies in SLE, these antibodies do not fix complement. Although they are most commonly found in drug induced lupus, they are also found in some cases of SLE, [[scleroderma]], [[rheumatoid arthritis]] and undifferentiated connective tissue disease. Many drugs are known to cause drug induced lupus and they produce various antigenic targets within the nucleosome that are often cross reactive with several histone proteins and DNA. Procainamide causes a form of drug-induced lupus that produces antibodies to the histone H2A and H2B complex.<ref>{{cite journal|last=Vasoo|first=S|title=Drug-induced lupus: an update.|journal=Lupus|date=2006|volume=15|issue=11|pages=757-61|pmid=17153847}}</ref><ref>{{cite journal|last=Katz|first=U|coauthors=Zandman-Goddard, G|title=Drug-induced lupus: an update.|journal=Autoimmunity reviews|date=2010 Nov|volume=10|issue=1|pages=46-50|pmid=20656071}}</ref> |
|||
| ⚫ | |||
Both [[anti-glycoprotein-210 antibodies|anti-glycoprotein-210]] (anti-gp210) and [[anti-p62 antibodies|anti-nucleoporin 62]] (anti-p62) antibodies are antibodies to components of the nuclear membrane and are found in [[primary biliary cirrhosis]] (PBC). Each antibody is present in approximately 25-30% of PBC. The antigens of both antibodies are constituents of the [[nuclear membrane]]. gp210 is a 200kd protein involved in anchoring components of the [[nuclear pore]] to the nuclear membrane. The p62 antigen is a 60kd nuclear pore complex.<ref>{{cite journal|last=Hu|first=T|coauthors=Guan, T; Gerace, L|title=Molecular and functional characterization of the p62 complex, an assembly of nuclear pore complex glycoproteins.|journal=The Journal of cell biology|date=1996 Aug|volume=134|issue=3|pages=589-601|pmid=8707840}}</ref><ref>{{cite journal|last=Mackay|first=IR|coauthors=Whittingham, S; Fida, S; Myers, M; Ikuno, N; Gershwin, ME; Rowley, MJ|title=The peculiar autoimmunity of primary biliary cirrhosis.|journal=Immunological reviews|date=2000 Apr|volume=174|pages=226-37|pmid=10807519}}</ref> |
Both [[anti-glycoprotein-210 antibodies|anti-glycoprotein-210]] (anti-gp210) and [[anti-p62 antibodies|anti-nucleoporin 62]] (anti-p62) antibodies are antibodies to components of the nuclear membrane and are found in [[primary biliary cirrhosis]] (PBC). Each antibody is present in approximately 25-30% of PBC. The antigens of both antibodies are constituents of the [[nuclear membrane]]. gp210 is a 200kd protein involved in anchoring components of the [[nuclear pore]] to the nuclear membrane. The p62 antigen is a 60kd nuclear pore complex.<ref>{{cite journal|last=Hu|first=T|coauthors=Guan, T; Gerace, L|title=Molecular and functional characterization of the p62 complex, an assembly of nuclear pore complex glycoproteins.|journal=The Journal of cell biology|date=1996 Aug|volume=134|issue=3|pages=589-601|pmid=8707840}}</ref><ref>{{cite journal|last=Mackay|first=IR|coauthors=Whittingham, S; Fida, S; Myers, M; Ikuno, N; Gershwin, ME; Rowley, MJ|title=The peculiar autoimmunity of primary biliary cirrhosis.|journal=Immunological reviews|date=2000 Apr|volume=174|pages=226-37|pmid=10807519}}</ref> |
||
Revision as of 20:18, 8 February 2013
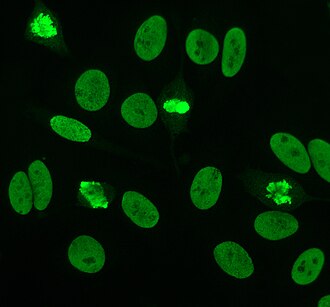
Anti-nuclear antibodies (ANAs, also known as anti-nuclear factor or ANF) are autoantibodies directed against contents of the cell nucleus.[1]
They are present in higher than normal numbers in autoimmune disease. The ANA test measures the pattern and amount of autoantibody which can attack the body's tissues as if they were foreign material. Autoantibodies are present in low titers in the general population, but in about 5% of the population, their concentration is increased, and about half of this 5% have an autoimmune disease.[citation needed]
ANA subtypes
Extractable nuclear antigens
Extractable nuclear antigens (ENA) are a group of autoantigens that were originally identified as antibody targets in patients with autoimmune disorders. They are termed ENA because they can be extracted from the cell nucleus with saline.[2][3] The ENAs consist of ribonucleoproteins and non-histone proteins, named by either the name of the patient who provided the prototype serum (Sm, Ro, La, Jo, Mi), or the name of the disease in which the antibodies were found (SS-A, SS-B, SS-C, Scl-70, PM, Scl-PM).[4]
Anti-Ro/SS-A and anti-La/SS-B

Anti-Ro and anti-La antibodies, also known as SS-A and SS-B, respectively, are commonly found in primary Sjögren's syndrome; an autoimmune disorder that affects the exocrine glands. The presence of both antibodies are found in 30-60% of Sjögren's syndrome, anti-Ro antibodies alone are found in 50-70% of Sjögren's syndrome and 30% of SLE with cutaneous involvement, and anti-La antibodies are rarely found in isolation.[5][6] Anti-Ro antibodies are also found less frequently in other disorders including autoimmune liver diseases, coeliac disease, autoimmune rheumatic diseases and cardiac neonatal lupus erythematosus.[7] During pregnancy, anti-Ro antibodies can cross the placenta and cause neonatal lupus in babies.[8] Anti-Ro antibodies are directed towards four proteins, which are 45kd, 52kd, 54kd and 60kd, and various hyRNAs. The 60kd DNA/RNA binding protein and 52kd T-cell regulatory protein have been intensely studied. Collectively, these proteins are part of a ribonucleoprotein (RNP) complex that associate with the hyRNAs, hY1-hY5. The La antigen is a 48kd transcription termination factor of RNA polymerase III, which associates with the Ro-RNP complex.[5][9][4][10]
Anti-Sm
Anti-Smith (Anti-Sm) antibodies are a very specific marker for SLE, with approximately 99% of patients with the antibodies having the disease. However, they are not sensitive, with around 20% of people with SLE having the antibodies. They are associated with central nervous system involvement, kidney disease, lung fibrosis and pericarditis in SLE, however they are not associated with disease activity. The antigens of the anti-Sm antibodies are the core units of the small nuclear ribonucleoproteins (snRNPs), termed A to G, and will bind to the U1, U2, U4, U5 and U6 snRNPs. Most commonly, the antibodies are specific for the B, B' and D units.[11][12]
Anti-nRNP/U1-RNP
Anti-nuclear ribonucleoprotein (anti-nRNP) antibodies, also known as anti-U1-RNP antibodies, are found in 30-40% of SLE patients. They are often found with anti-Sm antibodies, however they may be associated with different clinical associations. In addition to SLE, these antibodies are highly associated with mixed connective tissue disease. Anti-nRNP antibodies recognise the A and C core units of the snRNPs and because of this they primarily bind to the U1-snRNP.[11][13]
Anti-Scl-70/anti-topoisomerase I
Anti-Scl-70 antibodies are linked to scleroderma.[14]The sensitivity of the antibodies for scleroderma is approximately 34%, but is higher for patients with diffuse cutaneous involvement (40%), and lower in patients with limited cutaneous involvement (10%). The specificity of the antibodies is 98% and 99.6% in patients with other rheumatic diseases and normal individuals, respectively.[2][15] The antigenic target of anti-Scl-70 antibodies is topoisomerase I.[16]
Anti-Jo-1
Although anti-Jo-1 antibodies are often included with ANAs, they are actually antibodies to a cytoplasmic protein known as histidyl tRNA sythetase[3]. They are highly associated with polymyositis and dermatomyositis, and is rarely found in other connective tissue diseases. Around 20-40% of polymyositis patients are positive for Jo-1 antibodies and most will have both interstitial lung disease and HLA-DR3 and HLA-DRw52 human leukocyte antigen (HLA) markers; collectively known as Jo-1 syndrome.[17][11]
Anti-dsDNA

Anti-double stranded DNA antibodies (anti-dsDNA) are highly associated with SLE. They are a very specific marker for the disease, with some studies quoting nearly 100%. Data on sensitivity ranges from 25-85%. Anti-dsDNA antibody levels, knows as titres, correlate with disease activity in SLE; high levels indicate more active lupus. The presence of anti-dsDNA antibodies is also associated with lupus nephritis and evidence suggests that they may even cause this symptom. They may produce large immune complexes with DNA that bind to the glomerular basement membrane (GBM) or bind directly to antigens in the GBM. Both of these could initiate inflammation within the kidney, and also may fix complement. The antigen of anti-dsDNA antibodies is double stranded DNA[18][2][19]
Anti-histone antibodies
Anti-histone antibodies are found in the serum of up to 75-95% of drug induced lupus patients and 75% of idiopathic SLE. Unlike anti-dsDNA antibodies in SLE, these antibodies do not fix complement. Although they are most commonly found in drug induced lupus, they are also found in some cases of SLE, scleroderma, rheumatoid arthritis and undifferentiated connective tissue disease. Many drugs are known to cause drug induced lupus and they produce various antigenic targets within the nucleosome that are often cross reactive with several histone proteins and DNA. Procainamide causes a form of drug-induced lupus that produces antibodies to the histone H2A and H2B complex.[20][21]
Anti-gp210 and anti-p62
Both anti-glycoprotein-210 (anti-gp210) and anti-nucleoporin 62 (anti-p62) antibodies are antibodies to components of the nuclear membrane and are found in primary biliary cirrhosis (PBC). Each antibody is present in approximately 25-30% of PBC. The antigens of both antibodies are constituents of the nuclear membrane. gp210 is a 200kd protein involved in anchoring components of the nuclear pore to the nuclear membrane. The p62 antigen is a 60kd nuclear pore complex.[22][23]
Anti-centromere antibodies

Anti-centromere antibodies are associated with limited cutaneous systemic sclerosis, also known as CREST syndrome, primary billiary cirrhosis and proximal scleroderma.[24] There are six known antigens, which are all associated with the centromere; CENP-A to CENP-F. CENP-A is a 17kd histone H3-like protein. CENP-B is an 80kd DNA binding protein involved in the folding of heterochromatin. CENP-C is a 140kd protein involved in kinetochore assembly. CENP-D is a 50kd protein of unknown function, but may be homologous to another protein involved in chromatin condensation, RCC1. CENP-E is a 312kd protein from the kinesin motor protein family. CENP-F is a 367kd protein from the nuclear matrix that associates with the kinetochore in late G2 phase during mitosis. CENP-A, B and C antibodies are most commonly found (16-42% of systemic sclerosis patients) and are associated with Raynaud's phenomenon, telangiectasias, lung involvement and early onset in systemic sclerosis.[25][15][26]
Anti-sp100
Anti-sp100 antibodies are found in approximately 20-30% of primary biliary cirrhosis (PBC). They are found in few individuals without PBC, and therfore are a very specific marker of the disease. The sp100 antigen is found within nuclear bodies; large protein complexes in the nucleus that may have a role in cell growth and differentiation.[27]
ANA test
The presence of ANAs in blood can be confirmed by a screening test. Although there are many tests for the detection of ANAs, the most common tests used for screening are indirect immunofluoresence and enzyme-linked immunosorbent assay (ELISA).[28] Following detection of ANAs, various subtypes are determined.[2]
Indirect immunofluorescence

Indirect immunofluorescence is one of the most commonly used tests for ANAs. Typically, HEp-2 cells are used as a substrate to detect the antibodies in human serum. Microscope slides are coated with HEp-2 cells and the serum is incubated with the cells. If antibodies are present then they will bind to the antigens on the cells; in the case of ANAs, the antibodies will bind to the nucleus. These can be visualised by adding a fluorescent tagged (usually FITC or rhodopsin B) anti-human antibody that binds to the antibodies. The molecule will fluoresce when a specific wavelength of light shines on it, which can be seen under the microscope. Depending on the antibody present in the human serum and the localisation of the antigen in the cell, distinct patterns of fluorescence will be seen on the HEp-2 cells.[29][30] Levels of antibodies are analysed by performing dilutions on blood serum. An ANA test is considered positive if fluorescence is seen at a titre of 1:40/1:80. Higher titres are more clinically significant as low positives (≤1:160) are found in some healthy individuals.[31]
HEp-2
Until around 1975, when HEp-2 cells were introduced, animal tissue was used as the standard substrate for immunofluorescence.[6] HEp-2 cells are currently one of the most common substrates for ANA detection by immunofluorescence. They are superior to the previously used animal tissues because of their large size and the high rate of mitosis (cell division) in the cell line. This allows the detection of antibodies to mitosis-specific antigens, such as centromere antibodies. They also allow identification of anti-Ro antibodies, because acetone is used for fixation of the cells (other fixatives can wash the antigen away).[32]
There are many nuclear staining patterns seen on HEp-2 cells; homogeneous, speckled, nucleolar, nuclear membranous, centromeric, nuclear dot and pleiomorphic. The homogeneous pattern is seen when the condensed chromosomes and interphase chromatin stain. This pattern is associated with anti-dsDNA antibodies, antibodies to nucleosomal components and anti-histone antibodies. There are two speckled patterns; fine and coarse. The fine speckled pattern has fine nuclear staining with unstained metaphase chromatin, which is associated with anti-Ro and anti-La antibodies. The coarse staining pattern has coarse granular nuclear staining, caused by anti-U1-RNP and anti-Sm antibodies. The nucleolar staining pattern is associated with many antibodies including anti-Scl-70, anti-PM-Scl, anti-fibrillarin and anti-Th/To. Nuclear membrane staining appears as a fluorescent ring around the cell nucleus and are produced by anti-gp210 and anti-p62 antibodies. The centromere pattern shows multiple nuclear dots in interphase and mitotic cells, corresponding to the number of chromosomes in the cell. Nuclear dot patterns show between 13-25 nuclear dots in interphase cells and are produced by anti-sp100 antibodies. Pleiomorphic pattern is caused by antibodies to the proliferating cell nuclear antigen.[33][34][31][11]
Crithidia luciliae
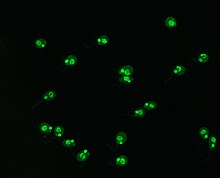
Crithidia luciliae are haemoflaggelate single celled protists. They are used as a substrate in immunofluorescence for the detection of anti-dsDNA antibodies. They possess an organelle known as the kinetoplast which is a large mitochondrion with a network of interlocking circular dsDNA molecules. After incubation with serum containing anti-dsDNA antibodies and fluorescent-labelled anti-human antibodies, the kinetoplast will fluoresce. The lack of other nuclear antigens in this organelle means that using C.luciliae as a substrate allows for the specific detection of anti-dsDNA antibodies.[2][35][36]
Associated diseases
The normal titer of ANA is 1:40 or less. Higher titers are indicative of an autoimmune disease. The presence of ANA is indicative of lupus erythematosus (present in 80-90% of cases), though they also appear in some other auto-immune diseases such as[citation needed] Sjögren's syndrome (60%), rheumatoid arthritis (30-40%), autoimmune hepatitis, scleroderma and polymyositis & dermatomyositis (30%), and various non-rheumatological conditions associated with tissue damage. Other conditions with high ANA titre include[citation needed] Addison disease, Idiopathic thrombocytopenic purpura (ITP), Hashimoto's, Autoimmune hemolytic anemia, Type I diabetes mellitus, Mixed connective tissue disorder (MCTD).
Sensitivity
The following table list the prevalence of different types of ANAs for different diseases, in this case what percentage of those with the disease have the ANA. Some ANAs appear in several types of disease, resulting in lower specificity of the test.
| ANA type | Target antigen | Sensitivity (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| SLE | Drug-induced LE | Diffuse systemic sclerosis | Limited systemic scleroderma | Sjögren syndrome | Inflammatory myopathy | MCTD | ||
| All ANAs (by indirect IF) |
Various | >95 | >95 | 70-90 | 70-90 | 50-80 | 40-60 | 95[37] |
| Anti-dsDNA | DNA | 40-60 | - | - | - | - | - | -[37] |
| Anti-Sm | Core proteins of snRNPs | 20-30 | - | - | - | - | - | -[37] |
| Anti-histone | Histones | 50-70 | 90[37] - 95 | - | - | - | - | -[37] |
| Anti Scl-70 | Type I topoisomerase | - | - | 28-70 | 10-18 | - | - | -[37] |
| Anti-centromere | Centromeric proteins | - | - | 22-26 | 90 | - | - | -[37] |
| Anti-snRNP70 | snRNP70 | 30[38]-40[37][38] | -[37] | 15[37] | 10[37] | -[37] | 15[37] | 90[37] |
| SS-A (Ro) | RNPs | 30-50 | - | - | - | 70-95 | 10 | -[37] |
| SS-B (La) | RNPs | 10-15 | - | - | - | 60-90 | - | -[37] |
| Jo-1 | Histidine-tRNA ligase | - | - | - | - | - | 25 | -[37] |
| - = less than 5% sensitivity
Unless else specified in boxes, then ref is:[38] | ||||||||
| ANA type | Target antigen | Specificity (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| SLE | Drug-induced LE | Diffuse systemic sclerosis | Limited systemic scleroderma | Sjögren syndrome | Inflammatory myopathy | MCTD | ||
| All ANAs (by indirect IF) |
Various | 57[39] | X | X | 54[39] | 52[39] | 63[39] | X |
| Anti-dsDNA | DNA | 97[39] | - | - | - | - | - | - |
| Anti-Sm | Core proteins of snRNPs | X | - | - | - | - | - | - |
| Anti-histone | Histones | X | X - | - | - | - | - | - |
| Anti Scl-70 | Type I topoisomerase | - | - | X | X | - | - | - |
| Anti-centromere | Centromeric proteins | - | - | X | X | - | - | - |
| Anti-snRNP70 | snRNP70 | X | - | X | X | - | X | X |
| SS-A (Ro) | RNPs | X | - | - | - | X | X | - |
| SS-B (La) | RNPs | X | - | - | - | X | - | - |
| Jo-1 | Histidine-tRNA ligase | - | - | - | - | - | X | - |
| - = less than 5% sensitivity
Unless else specified in boxes, then ref is:[38] | ||||||||
History
The LE cell was discovered in bone marrow in 1948 by Hargraves et al.[40] This was the first indication that processes affecting the cell nucleus were responsible for lupus erythematosus (LE).[citation needed] In the 1950s, progressively more sensitive and specific ANA serology tests became available.
See also
- ^ Antinuclear+Antibody at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- ^ a b c d e Kavanaugh A, Tomar R, Reveille J, Solomon DH, Homburger HA. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Arch Pathol Lab Med 2000;124:71-81. PMID 10629135.
- ^ a b Damoiseaux, JG (2006 Jan). "From ANA to ENA: how to proceed?". Autoimmunity reviews. 5 (1): 10–7. PMID 16338206.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Wenzel, J (2001 Dec). "Antibodies targeting extractable nuclear antigens: historical development and current knowledge". The British journal of dermatology. 145 (6): 859–67. PMID 11899137.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Hernández-Molina, G (2011 Jan). "The meaning of anti-Ro and anti-La antibodies in primary Sjögren's syndrome". Autoimmunity reviews. 10 (3): 123–5. PMID 20833272.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Kumar, Y (2009 Jan 2). "Antinuclear antibodies and their detection methods in diagnosis of connective tissue diseases: a journey revisited". Diagnostic pathology. 4: 1. PMID 19121207.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Defendenti, C (2011 Jan). "Clinical and laboratory aspects of Ro/SSA-52 autoantibodies". Autoimmunity reviews. 10 (3): 150–4. PMID 20854935.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Scofield, RH (2004 May 8). "Autoantibodies as predictors of disease". Lancet. 363 (9420): 1544–6. PMID 15135604.
{{cite journal}}: Check date values in:|date=(help) - ^ Deshmukh, US (2005 Nov). "Epitope spreading within lupus-associated ribonucleoprotein antigens". Clinical immunology (Orlando, Fla.). 117 (2): 112–20. PMID 16095971.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ben-Chetrit, E (1993 May). "The molecular basis of the SSA/Ro antigens and the clinical significance of their autoantibodies". British journal of rheumatology. 32 (5): 396–402. PMID 8495261.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c d von Mühlen, CA (1995 Apr). "Autoantibodies in the diagnosis of systemic rheumatic diseases". Seminars in arthritis and rheumatism. 24 (5): 323–58. PMID 7604300.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Lyons, R (2005 Jun). "Effective use of autoantibody tests in the diagnosis of systemic autoimmune disease". Annals of the New York Academy of Sciences. 1050: 217–28. PMID 16014537.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Benito-Garcia, E (2004 Dec 15). "Guidelines for immunologic laboratory testing in the rheumatic diseases: anti-Sm and anti-RNP antibody tests". Arthritis and rheumatism. 51 (6): 1030–44. PMID 15593352.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Jimenez, SA (2004 Jan 6). "Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis". Annals of internal medicine. 140 (1): 37–50. PMID 14706971.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Ho, KT (2003). "The clinical relevance of autoantibodies in scleroderma". Arthritis research & therapy. 5 (2): 80–93. PMID 12718748.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Guldner, HH (1986). "Scl 70 autoantibodies from scleroderma patients recognize a 95 kDa protein identified as DNA topoisomerase I.". Chromosoma. 94 (2): 132–8. PMID 2428564.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Schmidt, WA (2000). "Clinical and serological aspects of patients with anti-Jo-1 antibodies--an evolving spectrum of disease manifestations". Clinical rheumatology. 19 (5): 371–7. PMID 11055826.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Mok, CC (2003 Jul). "Pathogenesis of systemic lupus erythematosus". Journal of clinical pathology. 56 (7): 481–90. PMID 12835292.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Yung, S (2008 Feb). "Anti-DNA antibodies in the pathogenesis of lupus nephritis--the emerging mechanisms". Autoimmunity reviews. 7 (4): 317–21. PMID 18295737.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Vasoo, S (2006). "Drug-induced lupus: an update". Lupus. 15 (11): 757–61. PMID 17153847.
- ^ Katz, U (2010 Nov). "Drug-induced lupus: an update". Autoimmunity reviews. 10 (1): 46–50. PMID 20656071.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hu, T (1996 Aug). "Molecular and functional characterization of the p62 complex, an assembly of nuclear pore complex glycoproteins". The Journal of cell biology. 134 (3): 589–601. PMID 8707840.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Mackay, IR (2000 Apr). "The peculiar autoimmunity of primary biliary cirrhosis". Immunological reviews. 174: 226–37. PMID 10807519.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Kallenberg, CG (1990 Mar). "Anti-centromere antibodies (ACA)". Clinical rheumatology. 9 (1 Suppl 1): 136–9. PMID 2203592.
{{cite journal}}: Check date values in:|date=(help) - ^ Rattner, JB (1998 Jul). "Autoantibodies to components of the mitotic apparatus". Molecular biology reports. 25 (3): 143–55. PMID 9700050.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Renz, Harald. Autoimmune diagnostics. Berlin: De Gruyter. ISBN 978-3-11-022864-9.
- ^ Worman, HJ (2003 Jun). "Antinuclear antibodies specific for primary biliary cirrhosis". Autoimmunity reviews. 2 (4): 211–7. PMID 12848948.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Granito A, Muratori P, Quarneti C, Pappas G, Cicola R, Muratori L (2012). "Antinuclear antibodies as ancillary markers in primary biliary cirrhosis". Expert Review of Molecular Diagnostics. 12 (1): 65–74. doi:10.1586/erm.11.82. PMID 22133120.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Klein, Wulf B. Storch. Transl. by R. A. (2000). Immunofluorescence in clinical immunology : a primer and atlas. Basel [u.a.]: Birkhäuser. ISBN 3764361824.
- ^ González-Buitrago, JM (2006 Mar). "Present and future of the autoimmunity laboratory". Clinica chimica acta; international journal of clinical chemistry. 365 (1–2): 50–7. PMID 16126186.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Tozzoli, R (2002 Feb). "Guidelines for the laboratory use of autoantibody tests in the diagnosis and monitoring of autoimmune rheumatic diseases". American journal of clinical pathology. 117 (2): 316–24. PMID 11863229.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Keren, DF (2002 Jun). "Antinuclear antibody testing". Clinics in laboratory medicine. 22 (2): 447–74. PMID 12134471.
{{cite journal}}: Check date values in:|date=(help) - ^ Sack, U (2009 Jun). "Autoantibody detection by indirect immunofluorescence on HEp-2 cells". Deutsche medizinische Wochenschrift (1946). 134 (24): 1278–82. PMID 19499499.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Nesher, G (2001 Apr). "Anti-nuclear envelope antibodies: Clinical associations". Seminars in arthritis and rheumatism. 30 (5): 313–20. PMID 11303304.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Slater, NG (1976 Sep). "The Crithidia luciliae kinetoplast immunofluorescence test in systemic lupus erythematosus". Clinical and experimental immunology. 25 (3): 480–6. PMID 786521.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Shapiro, TA (1995). "The structure and replication of kinetoplast DNA". Annual review of microbiology. 49: 117–43. PMID 8561456.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e f g h i j k l m n o p q Table 6-2 in: Elizabeth D Agabegi; Agabegi, Steven S. (2008). Step-Up to Medicine (Step-Up Series). Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 0-7817-7153-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Table 5-9 in: Mitchell, Richard Sheppard; Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson (2007). Robbins Basic Pathology. Philadelphia: Saunders. ISBN 1-4160-2973-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) 8th edition. - ^ a b c d e Colglazier, CL (2005 Feb). "Laboratory testing in the rheumatic diseases: a practical review". Southern medical journal. 98 (2): 185–91. PMID 15759949.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hargraves M, Richmond H, Morton R. Presentation of two bone marrow components, the tart cell and the LE cell. Mayo Clin Proc 1948;27:25–28.
External links
- Site with unique immunofluorescence images and slides -organ and non-organ specific
- Antinuclear+antibodies at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
