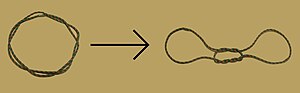Obsolete models of DNA structure: Difference between revisions
Ycxuwiki2012 (talk | contribs) |
Ycxuwiki2012 (talk | contribs) |
||
| Line 35: | Line 35: | ||
In 1963, [[John Cairns (biochemist)|John Cairns]] published [[autoradiograph]]s of the ''E. coli'' chromosome, showing that it was a single circular molecule that is replicated at a moving locus - the "[[replication fork]]" - at which both new DNA strands are being synthesized.<ref name="Cairns_1963">{{cite journal | last = Cairns | first = J. | title = The bacterial chromosome and its manner of replication as seen by autoradiography | journal = J. Mol. Biol. | volume = 6 | issue = | pages = 208–13 | year = 1963 | month = March | pmid = 14017761 | doi = }}</ref> Cairns, at that time, recognized a topological problem in that the helical twists between the daughter strands would have to be removed if the strands are to separate during cell replication. He proposed that the twists were unwound by means of a hypothetical "swivel" around which the chromosome would be free to rotate as necessary for the un-winding and re-winding operations.<ref name="cairns2">{{cite journal | author = Cairns J | year = 1963 | title = The chromosome of Escherichia coli | url = | journal = Cold Spring Harbor Symp Quant Biol | volume = 28 | issue = | pages = 43–46 | doi = 10.1101/SQB.1963.028.01.011 }}</ref> However, such a structure would have to have about 400,000 helical twists in the Watson–Crick model, each of which would have to be removed in as little as 20 minutes, if the strands are to separate during cell replication. This leads to the apparent need of the chromosome - or at least some part of it - to be spinning at speeds up to 6900–9000 rpm throughout the life of the bacterial cell.<ref>{{cite journal|first=F.|last=Górski|title=Critical review of the mechanisms separating the strands of DNA double helices during cell division|journal=Folia Biologica (Krakow)|year=1975|volume=23|issue=1|pages=81–111|pmid=1157978}}</ref> |
In 1963, [[John Cairns (biochemist)|John Cairns]] published [[autoradiograph]]s of the ''E. coli'' chromosome, showing that it was a single circular molecule that is replicated at a moving locus - the "[[replication fork]]" - at which both new DNA strands are being synthesized.<ref name="Cairns_1963">{{cite journal | last = Cairns | first = J. | title = The bacterial chromosome and its manner of replication as seen by autoradiography | journal = J. Mol. Biol. | volume = 6 | issue = | pages = 208–13 | year = 1963 | month = March | pmid = 14017761 | doi = }}</ref> Cairns, at that time, recognized a topological problem in that the helical twists between the daughter strands would have to be removed if the strands are to separate during cell replication. He proposed that the twists were unwound by means of a hypothetical "swivel" around which the chromosome would be free to rotate as necessary for the un-winding and re-winding operations.<ref name="cairns2">{{cite journal | author = Cairns J | year = 1963 | title = The chromosome of Escherichia coli | url = | journal = Cold Spring Harbor Symp Quant Biol | volume = 28 | issue = | pages = 43–46 | doi = 10.1101/SQB.1963.028.01.011 }}</ref> However, such a structure would have to have about 400,000 helical twists in the Watson–Crick model, each of which would have to be removed in as little as 20 minutes, if the strands are to separate during cell replication. This leads to the apparent need of the chromosome - or at least some part of it - to be spinning at speeds up to 6900–9000 rpm throughout the life of the bacterial cell.<ref>{{cite journal|first=F.|last=Górski|title=Critical review of the mechanisms separating the strands of DNA double helices during cell division|journal=Folia Biologica (Krakow)|year=1975|volume=23|issue=1|pages=81–111|pmid=1157978}}</ref> |
||
==Rodley non-helical structure proposed== |
<ref></ref>==Rodley non-helical structure proposed== |
||
[[File:Rodley Side-By-Side DNA structure.ogv|thumb|300px|A video of the Rodley side-by-side structure</div>|alt=Movie showing a single 360º rotation of a 30-base-pair length of the Rodley Side-By-Side structure.<ref name="rodley2">Biegeleisen, K., 2005. Rodley Side-By-Side Structure. Protein Data Bank, http://www.rcsb.org/, accession number 2AW8.</ref>]] |
[[File:Rodley Side-By-Side DNA structure.ogv|thumb|300px|A video of the Rodley side-by-side structure</div>|alt=Movie showing a single 360º rotation of a 30-base-pair length of the Rodley Side-By-Side structure.<ref name="rodley2">Biegeleisen, K., 2005. Rodley Side-By-Side Structure. Protein Data Bank, http://www.rcsb.org/, accession number 2AW8.</ref>]] |
||
Revision as of 18:24, 28 March 2013
This article needs attention from an expert in Molecular and Cellular Biology. The specific problem is: more sources are needed documenting the majority viewpoint on DNA structure. (May 2012) |
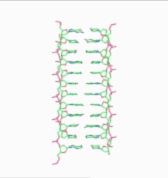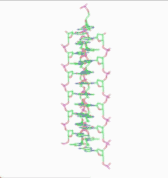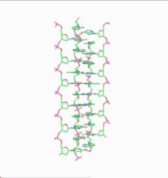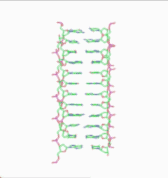
In the history of molecular biology, non-helical or "side-by-side" models of DNA were proposed in the 1970s as a challenge to the standard double-helical model. The non-helical models attempted to solve problems relating to the topology of circular DNA chromosomes during replication. These theories were briefly considered seriously as a minority viewpoint,[1] but they were later largely rejected due to X-ray crystallography of DNA duplexes and later the nucleosome core particle, as well as the discovery of topoisomerases, and these non-double-helical models are not currently accepted by the mainstream scientific community.[2]
Terminology
The strands of a helical DNA duplex, if covalently closed into a circular structure, are topologically linked, and thus cannot be separated without breaking one or both strands. This sort of structure is referred to as "plectonemic". In circular DNA replication, these twists must be removed for the daughter strands to separate. In contrast, the strands of a non-helical DNA duplex would be topologically non-linked, and can be separated without strand breakage. This sort of structure is referred to as "paranemic".[3][4]
Note that the term "non-helical" refers to net helicity - there can be no helical twist at all, as in the figure at the top of this article, or there could be an equal number of right-handed and left-handed twists, as in the Rodley structure below. The abbreviation "TN", to be used to refer to any DNA structure whose strands are topologically non-linked, has been proposed.[3]
Historical background
Proposal of Watson–Crick helical structure
The double-helix model of DNA structure was first published in the journal Nature by James D. Watson and Francis Crick in 1953[5] with further details in 1954.[6]) Their work was based upon the crucial X-ray diffraction image of DNA - labeled as "Photo 51" - from Rosalind Franklin in 1952,[7] followed by her more highly clarified DNA image with Raymond Gosling,[8][9] Maurice Wilkins, Alexander Stokes, and Herbert Wilson,[10] as well as base-pairing chemical and biochemical information by Erwin Chargaff.[11][12] Wilkins and colleagues also reported on X-ray patterns of the state of in vivo B-DNA in partially oriented salmon sperm heads.[10] Crick, Wilkins, and Watson each received one-third of the 1962 Nobel Prize in Physiology or Medicine for their contributions to this discovery.[13] Franklin, whose breakthrough X-ray diffraction data was used to formulate the DNA structure, died in 1958, and thus was ineligible to be nominated for a Nobel Prize.
Discovery of circular DNA
In 1963, John Cairns published autoradiographs of the E. coli chromosome, showing that it was a single circular molecule that is replicated at a moving locus - the "replication fork" - at which both new DNA strands are being synthesized.[14] Cairns, at that time, recognized a topological problem in that the helical twists between the daughter strands would have to be removed if the strands are to separate during cell replication. He proposed that the twists were unwound by means of a hypothetical "swivel" around which the chromosome would be free to rotate as necessary for the un-winding and re-winding operations.[15] However, such a structure would have to have about 400,000 helical twists in the Watson–Crick model, each of which would have to be removed in as little as 20 minutes, if the strands are to separate during cell replication. This leads to the apparent need of the chromosome - or at least some part of it - to be spinning at speeds up to 6900–9000 rpm throughout the life of the bacterial cell.[16]
Cite error: There are <ref> tags on this page without content in them (see the help page).==Rodley non-helical structure proposed==
In 1976, Gordon Rodley and his co-workers published the first non-helical DNA structure, which they named the "side-by-side" (SBS) structure.[18] A similar model was published a few months later by Sasisekharan and his associates.[19][20] The SBS structure was hypothetical, and was not based primarily upon physical evidence. It was created to provide a theoretical solution to the angular momentum problem in DNA replication, which Rodley believed to be otherwise unsolvable.
The SBS structure is a modification of the original Watson-Crick structure where, instead of one fully right-handed helical twist every 10 base pairs, there is only a half-twist of 5 base pairs’ length, followed immediately by a half-twist in the opposite direction (that is, a left-handed half-twist, also of 5 base pairs’ length). This alternating pattern of right- and left-handed half-twists is repeated throughout the length of the chromosome which, topologically speaking, would have no net twists. Such a chromosome does not need to either spin or unwind during replication. Rodley in later published work makes it clear that it was not the intention of his team that the model utilized alternating half turns of opposite helical sense, but lengths of DNA having alternating helical senses deployed in the available space along the DNA molecule that would not exactly be half turns.[21]
Cite error: There are <ref> tags on this page without content in them (see the help page).==Experimental studies of circular DNA structure==
One key observation in support of a double-helical structure is that the individual single strands of small circular viral and plasmid DNA are inseparable under the usual types of denaturation conditions, supporting a plectonemic, i.e., twisted structure whose strands are topologically locked together. While human and bacterial chromosomes are far too large to isolate intact, but rather break into innumerable small fragments when extracted from their respective cells, there exist many species of small viral and plasmid chromosomes which because of their small sizes are stable in solution, and remain intact even with vigorous handling. Early studies quickly revealed that the strands of duplex circular chromosomes do not separate when subjected to conditions which readily denature linear DNA.[22] For example, if calf thymus DNA (a heterogeneous collection of linear fragments) is merely boiled, the strands separate, and when slowly cooled, they re-anneal. In contrast, when the circular chromosomes of such organisms as the replicative form (RF) of the bacteriophage φX174, or the mammalian virus polyoma, are boiled, nothing at all happens to the native structure - they are completely resistant to thermal denaturation. Without a doubt, the single most skepticism-inspiring aspect of non-helical DNA theory was the failure of the strands of circular chromosomes to separate under conditions where the strands of linear DNA would readily do so. This was generally regarded as evidence that the strands were plectonemically wound together (i.e., topologically linked).
The publication of the Rodley structure produced a small but significant ripple of interest in the molecular biological community. However, the level of interest was sufficiently high so that Crick - by that time one of the world’s most influential scientists - publicly suggested that the SBS structure (whose existence he doubted) should be laid to rest by intentionally creating it. Then, he reasoned, its properties could be ascertained, and then shown to be abnormal with respect to the known properties of native DNA.[23] An investigation by Stettler et al. followed closely on the heels of the Crick suggestion. They isolated the intact circular single strands of a circular duplex viral chromosome and then re-annealed them, giving a product they declared to be a "base-paired circular duplex with no net helical twist". If so, this would in fact be the Rodley "side-by-side" structure. They introduced a new terminology for this product, calling it "Form V" (extending the nomenclature of Forms I–IV used in sedimentation studies of DNA), and then went on to demonstrate that its electrophoretic mobility was clearly different from that of the native chromosome.[24]

The results of this investigation were not accepted by some authors, who agreed that it was established satisfactorily that "Form V" is different from native DNA, but did not establish that "Form V" has the SBS structure. While the strands of native circular DNA are known not to be separable under common denaturing conditions, in 1996 Tai Te Wu reported having found conditions under which fully intact single strands of two different circular duplex plasmids could be separated.[25] Wu believed that, because these plasmids have D-loops with DNA–RNA duplexes from RNA trascription only on their sense side, and since DNA–RNA interactions are stronger than DNA–DNA interactions,[26] the sense and anti-sense strands would thus have different electrophoretic mobilities. He employed a very low voltage, so that the electrophoresis required 48 hours to go to completion. DNA sequencing then indicated that each of the two bands were enriched in one of the two strands of the chromosome.
In 2009, an ambidextrous double helix DNA model was proposed by Xu based on his topological investigation ([27] Xu YC, Finding of a zero linking number topoisomer. 2009. B.B.A. 1790, 126-133.
[28] Xu YC, Replication demands an amendment of the double helix.29—56 in , Ed. by H. Seligmann DNA Replication---recent advances, InTech. 2011), which reconcile the disparity of arguments between two groups. Different from most of those SBS models, its main concept is that the two strands inside the double helix are winding ambidextrously, rather than plectonemically. Hence all the evidences favorable to the Watson - Crick Model are still valid. At the same time, it strongly insists that there is a lot of left-handed DNA co-existing with the right-handed DNA. The main evidence comes from the finding of a zero linking number topoisomer, which is topologically impossible if the two strands of a covalently closed circular DNA are always winding in right-handed direction as claimed in the Watson-Crick Model. According to this bidirectional model, the discovery of topoisomerases becomes a favorable evidence to take "the sting" out of the topological objection from the classical double helix model. And the function of the slow reaction rate of gyrase (6 reactions per minute) in the replisome located at replication fork can get a perfect explanation for the quick DNA replication rate (1kb per second or 6,000 bases per minute). Although the proposed amendment to the Watson-Crick Model is just change winding direction of two strands from the yes/no to either/or, it may have great consequences in the understanding of DNA topology and the mechanism of DNA replication. This amendment actually is required by the established evidences of semi-conservative bi-directional DNA replication in E.coli and many other bacteria.
Other experimental developments
This section needs expansion. You can help by adding to it. (June 2012) |
The discovery of topoisomerases, enzymes that can change the linking number of circular nucleic acids and thus "unwind" the replicating bacterial chomosome, was largely seen by the mainstream scientific community to satisfy the topological objection to the Watson–Crick helical structure. The later X-ray crystallography data for the nucleosome, which showed a helical DNA structure wrapped around the protein nucleosome core particle, was considered to provide further affirmation for the existence of the helical structure in vivo. Non-double-helical models are not currently accepted by the mainstream scientific community.[2]
Enzymes capable of unwinding and rewinding DNA helical twists, namely topoisomerases and gyrases, are essential for life, and necessary for DNA replication in various in vitro synthesis systems.
References
- ^ Stokes, T. D. (1982). "The double helix and the warped zipper—an exemplary tale". Social Studies of Science. 12 (2): 207–240. doi:10.1177/030631282012002002.
{{cite journal}}:|access-date=requires|url=(help) - ^ a b Gautham, N. (25 May 2004). "Response to "Variety in DNA secondary structure"" (PDF). Current Science. 86 (10): 1352–1353. Retrieved 28 November 2012.
However, the discovery of topoisomerases took "the sting" out of the topological objection to the plectonaemic double helix. The more recent solution of the single crystal X-ray structure of the nucleosome core particle showed nearly 150 base pairs of the DNA (i.e. about 15 complete turns), with a structure that is in all essential respects the same as the Watson–Crick model. This dealt a death blow to the idea that other forms of DNA, particularly double helical DNA, exist as anything other than local or transient structures.
- ^ a b Biegeleisen K (2002). "Topologically non-linked circular duplex DNA". Bull. Math. Biol. 64 (3): 589–609. doi:10.1006/bulm.2002.0288. PMID 12094410.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Delmonte, C. S.; Mann, L. R. B. "Variety in DNA secondary structure" (PDF). Current Science. 85: 1564–1570.
- ^ James D. Watson and Francis Crick (1953). "A structure for deoxyribose nucleic acid" (PDF). Nature. 171 (4356): 737–8. Bibcode:1953Natur.171..737W. doi:10.1038/171737a0. PMID 13054692.
- ^ Crick, Francis, and James D. Watson (1954). "The Complementary Structure of Deoxyribonucleic Acid" (PDF). Proceedings of the Royal Society of London. 223, Series A: 80–96.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Secret of Photo 51". NOVA. PBS.
- ^ http://www.nature.com/nature/dna50/franklingosling.pdf
- ^ The Structure of the DNA Molecule
- ^ a b Wilkins MHF, Stokes AR, Wilson HR (1953). "Molecular Structure of Deoxypentose Nucleic Acids" (PDF). Nature. 171 (4356): 738–740. Bibcode:1953Natur.171..738W. doi:10.1038/171738a0. PMID 13054693.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "NatWilk" was defined multiple times with different content (see the help page). - ^ Magasanik B, Vischer E, Doniger R, Elson D, Chargaff E (1950). "The separation and estimation of ribonucleotides in minute quantities". J Biol Chem. 186 (1): 37–50. PMID 14778802.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Chargaff E (1950). "Chemical specificity of nucleic acids and mechanism of their enzymatic degradation". Experientia. 6 (6): 201–209. doi:10.1007/BF02173653. PMID 15421335.
- ^ "Nobel Prize - List of All Nobel Laureates".
- ^ Cairns, J. (1963). "The bacterial chromosome and its manner of replication as seen by autoradiography". J. Mol. Biol. 6: 208–13. PMID 14017761.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Cairns J (1963). "The chromosome of Escherichia coli". Cold Spring Harbor Symp Quant Biol. 28: 43–46. doi:10.1101/SQB.1963.028.01.011.
- ^ Górski, F. (1975). "Critical review of the mechanisms separating the strands of DNA double helices during cell division". Folia Biologica (Krakow). 23 (1): 81–111. PMID 1157978.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Biegeleisen, K., 2005. Rodley Side-By-Side Structure. Protein Data Bank, http://www.rcsb.org/, accession number 2AW8.
- ^ Rodley GA, Scobie RS, Bates RH, Lewitt RM (1976). "A possible conformation for double-stranded polynucleotides". Proc. Natl. Acad. Sci. U.S.A. 73 (9): 2959–63. Bibcode:1976PNAS...73.2959R. doi:10.1073/pnas.73.9.2959. PMC 430891. PMID 1067594.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sasisekharan V, Pattabiraman N & Gupta G (1976). Curr Sci 45, 779-783. Current Science home page: http://cs-test.ias.ac.in/cs/index.php. Free PDF download at http://cs-test.ias.ac.in/cs/Downloads/article_18154.pdf.
- ^ Sasisekharan V, Pattabiraman N, Gupta G (1978). "Some implications of an alternative structure for DNA". Proc. Natl. Acad. Sci. U.S.A. 75 (9): 4092–6. Bibcode:1978PNAS...75.4092S. doi:10.1073/pnas.75.9.4092. PMC 336057. PMID 279899.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1007/BF02703272, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1007/BF02703272instead. - ^ Vinograd J, Lebowitz J, Radloff R, Watson R, Laipis P (1965). "The twisted circular form of polyoma viral DNA". Proc. Natl. Acad. Sci. U.S.A. 53 (5): 1104–11. Bibcode:1965PNAS...53.1104V. doi:10.1073/pnas.53.5.1104. PMC 301380. PMID 4287964.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Crick FH, Wang JC, Bauer WR (1979). "Is DNA really a double helix?" (PDF). J. Mol. Biol. 129 (3): 449–57. doi:10.1016/0022-2836(79)90506-0. PMID 458852.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Stettler UH, Weber H, Koller T, Weissmann C (1979). "Preparation and characterization of form V DNA, the duplex DNA resulting from association of complementary, circular single-stranded DNA". J. Mol. Biol. 131 (1): 21–40. doi:10.1016/0022-2836(79)90299-7. PMID 490644.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wu R, Wu T (1996). "A novel intact circular dsDNA supercoil". Bull. Math. Biol. 58 (6): 1171–85. doi:10.1007/BF02458388. PMID 8953261.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Casey J, Davidson N (1977). "Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide". Nucleic Acids Res. 4 (5): 1539–52. doi:10.1093/nar/4.5.1539. PMC 343772. PMID 19730.
- ^ 27
- ^ 28