Fuel cell: Difference between revisions
No edit summary |
No edit summary |
||
| Line 275: | Line 275: | ||
== External links == |
== External links == |
||
*[http://www.fuelcells.org Fuel Cells 2000] |
*[http://www.fuelcells.org Fuel Cells 2000] <html> |
||
<a href="http://www.jmfuelcells.com/html/about_jmfc.html">Johnson Matthey Fuel Cells</a>[] |
<a href="http://www.jmfuelcells.com/html/about_jmfc.html">Johnson Matthey Fuel Cells</a>[] |
||
* [http://www.bigs.de/en/shop/htm/bz01.html BIGS: Fuel Cell Animation] |
</html>* [http://www.bigs.de/en/shop/htm/bz01.html BIGS: Fuel Cell Animation] |
||
* [http://www.eere.energy.gov/hydrogenandfuelcells/fuelcells/fc_types.html EERE: Fuel Cell Types] |
* [http://www.eere.energy.gov/hydrogenandfuelcells/fuelcells/fc_types.html EERE: Fuel Cell Types] |
||
* [http://www.eere.energy.gov/hydrogenandfuelcells/ EERE: Hydrogen, Fuel Cells and Infrastructure Technologies Program] |
* [http://www.eere.energy.gov/hydrogenandfuelcells/ EERE: Hydrogen, Fuel Cells and Infrastructure Technologies Program] |
||
Revision as of 20:55, 25 May 2006
A fuel cell is an electrochemical energy conversion device similar to a battery, but differing from the latter in that it is designed for continuous replenishment of the reactants consumed; i.e. it produces electricity from an external supply of fuel and oxygen as opposed to the limited internal energy storage capacity of a battery. Additionally, the electrodes within a battery react and change as a battery is charged, or discharged, whereas a fuel cell's electrodes are catalytic and relatively stable.
Typical reactants used in a fuel cell are hydrogen on the anode side and oxygen on the cathode side (a hydrogen cell). Usually, reactants flow in and reaction products flow out. Virtually continuous long-term operation is feasible as long as these flows are maintained.
Technology

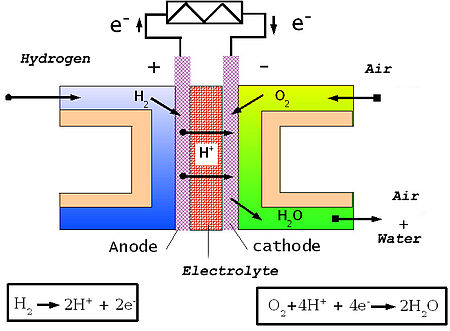
In the archetypal example of a hydrogen/oxygen proton-exchange membrane (or "polymer electrolyte") fuel cell (PEMFC), a proton-conducting polymer membrane, (the electrolyte), separates the anode and cathode sides.
On the anode side, hydrogen diffuses to the anode catalyst where it dissociates into protons and electrons. The protons are conducted through the membrane to the cathode, but the electrons are forced to travel in an external circuit (supplying power) because the membrane is electrically insulating. On the cathode catalyst, oxygen molecules react with the electrons (which have travelled through the external circuit) and protons to form water. In this example, the only waste product is water vapor and/or liquid water.
In addition to pure hydrogen, there are hydrogen-carrying fuels for fuel cells, including diesel, methanol (see DMFC) and chemical hydrides, the waste product with these type of fuels is carbon dioxide.
Voltage
A typical fuel cell produces about 0.8 volts. To create enough voltage, the cells are layered and combined in series and parallel into the so called "Fuel Cell Stack." The number of cells used is usually greater than 45 and varies with design.
Materials

The materials used in fuel cells differ by type. See Types of fuel cells.
1.The electrode/bipolar plates are usually made of metal, nickel or carbon nanotubes, and are coated with a catalyst (like platinum or palladium) for higher efficiency. 2.carbon paper. 3/4.The electrolyte could be ceramic or a membrane.
Example: The construction of the Low temperature fuel cell PEMFC:
- Bipolarplate as electrode with in-milled gas channel structure, fabricated from conductive plastics (enhanced with carbon nanotubes for more conductivity);
- Porous carbon papers;
- Reactive layer, usually on the polymer membrane applied.
- polymer membrane
- Same as 3);
- Same as 2);
- Same as 1)
Fuel cell design issues
- Costs. In 2002, typical cells had a catalyst content of USD 1000 per kW of electric power output, which is expected, by 2007, to be reduced to USD 30 per kW [1]. Ballard's success is a catalyst enhanced with carbon silk with a 30% reduction (1 mg/cm2 to 0.7 mg/cm2) of platinum without reduction in performance (2005)[2].
- The production costs a.c.a MEA (membrane electrode assembly) of the PEM (proton exchange membrane). The Nafion® membrane, -/+ 400,= Euro/m2, followed by the Toyota PEM and 3M PEM membrane are replaced by the ITM Power membrane, resulting in a price of -/+ 4 Euro/m2 (2004). This new membrane is a Hydrocarbon-polymer. One of the bigger companies is using Solupor® (a porous polyethylene film)[3].
- Water management in (PEMFC's). In this type of fuel cell, the membrane must be hydrated, requiring water to be evaporated at precisely the same rate that it is produced. If water is evaporated too quickly, the membrane dries, resistance across it increases, and eventually it will crack, creating a gas "short circuit" where hydrogen and oxygen combine directly, generating heat that will damage the fuel cell. If the water is evaporated too slowly, the electrodes will flood, preventing the reactants from reaching the catalyst and stopping the reaction. Methods to dispose of the excess water are being developed by fuel cell companies.
- Flow control. Just as in a combustion engine, a steady ratio between the reactant and oxygen is necessary to keep the fuel cell operating efficiently.
- Temperature management. The same temperature must be maintained throughout the cell in order to prevent destruction of the cell through thermal loading. (See Watercooling).
- Durability, lifetime, and special requirements for some type of cells. Stationary applications typically require more than 40,000 hours of reliable operation at a temperature of -35°C to 40°C, while automotive fuel cells require a 5,000 hour lifespan (the equivalent of 150,000 miles) under extreme temperatures. (See: Hydrogen vehicle. Status 2005: lifetime reached.) Automotive engines must also be able to start reliably at -30°C and have a high power to volume ratio (typically 2.5kW per liter).
- Limited CO tolerance of the anode.
Types of fuel cells
| Name | Electrolyte | Range | Working temp. |
elec. eff. | Status |
|---|---|---|---|---|---|
| Reversible fuel cell | Learning kit | ||||
| Blue energy | polyethylene membrane | Up to 250KW | Research | ||
| MFC - Biological fuel cell | |||||
| Zinc fuel cell ('Air' fuel cell) | |||||
| Redox fuel cell | Liquid electrolytes with redox shuttle and polymer membrane | Research | |||
| AFC - Alkaline fuel cell | alkaline solution | 10 to 100 kW | under 80°C | Cell: 60–70% System: 62% | Commercial/Research |
| PEM FC - Proton exchange membrane fuel cell | polymer membrane (ionomer) | 0,1 to 500 kW | 70–200 °C, | Cell: 50–70 % System: 30–50 % | Commercial/Research |
| DBFC - Direct borohydride fuel cell | alkaline solution: NaOH | 70 °C | Research | ||
| FAFC - Formic acid fuel cell | formic acid | 90–120 °C | Research | ||
| DMFC - Direct methanol fuel cell | polymer membrane | mW to 100 kw | 90–120 °C | Cell: 20–30 % | Commercial/Research |
| DEFC - Direct-ethanol fuel cell | Research | ||||
| PAFC - Phosphoric-acid fuel cell | phosphoric acid | Up to 10 MW | 200 °C | Cell: 55 % System: 40 % | Commercial/Research |
| MCFC - Molten carbonate fuel cell | molten alkaline-carbonate | 100 MW | 650 °C | Cell: 55 % System: 47 % | Commercial/Research |
| PCFC - Protonic ceramic fuel cell | ceramic | 700 °C | Research | ||
| SOFC - Solid oxide fuel cell | oxide ceramic electrolyte | Up to 100 MW | 800–1000 °C | Cell: 60–65 % System: 55–60 % | Commercial/Research |
Efficiency
Fuel cells are not constrained by the maximum Carnot cycle efficiency as combustion engines are, because they do not operate with a thermal cycle. Consequently, they can have very high efficiencies in converting chemical energy to electrical energy.
Definitions of Efficiency
In physics, there are many definitions of efficiency, and different branches of engineering have their customary definitions. As fuel cells involve many branches of science, it is common to misunderstand the actual meaning of what is termed "efficiency".
Mechanical engineers often use the first-law efficiency, defined as the ratio of useful energy obtained by a certain process divided by the maximum development of heat of that process when no work is extracted (the enthalpy). However, in thermodynamics, the maximum work that can be extracted by a process is the Gibbs free energy: given the standard Gibbs free energy and enthalpy of the reaction, this efficiency would therefore be limited by:
(for water in liquid form)
However, since is larger than work extracted by any real process, that ratio will always be less than about 87%. Since the totality of is physically unattainable, some engineers (such as chemical engineers) and researchers prefer the second-law efficiency, defined as:
where is the Gibbs free energy of the reactants in the form they are available in (e.g., gas at high pressure contains more usable energy than at atmospheric pressure; see also exergy). The second-law efficiency has the advantage of giving a meaningful roof for efficiency values, since an ideal process would attain exactly ; however, even in much of the scientific literature, the first-law efficiency is often used.
Typical values
A fuel cell typically converts the chemical energy of its fuel into electricity with an efficiency of about 50%. The efficiency is however very dependent on the current through the fuel cell: as a general rule, the more current drawn, the lower the efficiency. For a hydrogen cell the second-law efficiency is equal to cell voltage divided by 1.23 volts, when operating at standard conditions. This voltage varies with fuel used, and quality and temperature of the cell. A cell running at 0.6V has therefore an efficiency of about 50%, meaning that 50% of the available energy content of the hydrogen is converted into electrical energy; the remaining 50% will be converted into heat. The difference between enthalpy and Gibbs free energy (that cannot be recovered) will also appear as heat.
Plant-to-Wheel
It is also important to take losses due to production, transportation, and storage into account. Fuel cell vehicles running on compressed hydrogen may have a power-plant-to-wheel efficiency of 22% if the hydrogen is stored as high-pressure gas, and 17% if it is stored as liquid hydrogen (efficiency of Hydrogen Fuel Cell, Diesel-SOFC-Hybrid and Battery Electric Vehicles).[citation needed]
Round-trip Efficiency
Fuel cells cannot store energy like a battery, but in some applications, such as stand-alone power plants based on discontinuous sources (solar, wind power), they are combined with electrolyzers and storage systems to form an energy storage system. The overall efficiency (electricity to hydrogen and back to electricity) of such plants (known as round-trip efficiency) is between 30 and 50%, depending on conditions.[citation needed]
Whereas a much cheaper lead-acid battery might return about 90 percent, the electrolyser/fuel cell system can store indefinite quantities of hydrogen, and is therefore better suited for long-term storage.
Combined Heat and Power
In "combined heat and power" applications, a fuel cell is placed in a location where heat is also needed. A lower fuel-to-electricity conversion efficiency is tolerated (typically 15-20%), because most of the energy not converted into electricity is utilized as heat. Some heat is lost with the exhaust gas just as in a normal furnace, so the combined heat and power efficiency is still lower than 100%, typically around 80%. In terms of exergy however, the process is inefficient, and one could do better by maximizing the electricity generated and then using the electricity to drive a heat pump.
Fuel cell applications
Fuel cells are very useful as power sources in remote locations, such as spacecraft, remote weather stations, large parks, rural locations, and in certain military applications. A fuel cell system running on hydrogen can be compact, lightweight and has no major moving parts.
A new application is combined heat and power (CHP) for family home, office buildings and factories. This type of system generates constant electric power (selling excess power back to the grid when it is not consumed), and at the same time produce hot air and water from the waste heat. Phosphoric-acid fuel cells (PAFC) comprise the largest segment of existing CHP products worldwide and can provide combined efficiencies close to 80% (45-50% electric + remainder as thermal). The largest manufacturer of PAFC fuel cells is UTC Power, a division of United Technologies Corporation. Molten-carbonate fuel cells have also been installed in these applications, and Solid-oxide fuel cell prototypes exist.
However, since electrolyzer systems do not store fuel in themselves, but rather rely on external storage units, they can be successfully applied in large-scale energy storage, rural areas being one example. In this application, batteries would have to be largely oversized to meet the storage demand, but fuel cells only need a larger storage unit (typically cheaper than an electrochemical device).
One such pilot program exists on Stuart Island off the State of Washington. There the Stuart Island Energy Initiative has built a complete system by which solar panels generate the current to run several electrolyzers whose hydrogen is stored in a 500 gallon tank at 150-200 PSI. The hydrogen is then used to run a 48V ReliOn hydrogen fuel cell that provides full electric back-up to the residential site on this off the grid island (see external link to SIEI.ORG).
Protium, a rock band originating at Ponaganset High School in Glocester, RI was the world's first hydrogen fuel cell powered band. The band was powered by a 1kw Airgen Fuelcell from Ballard Power systems. The band has played at a number of fuel cell advocasy events inluding the Connecticut CEP, and the 2003 Fuel Cell Seminar in Miami beach, FL.
Plug Power Inc. is a major player in the design, development and manufacture of PEM fuel cells for stationary applications, including products aimed at telecommunication, prime power, and combined heat and power (CHP) applications.
Suggested applications
- Base load power plants
- Electric and hybrid vehicles.
- Auxiliary power
- Off-grid power supply
Hydrogen vehicles, boats and refuelling

and Hydrogen highway.
The first hydrogen refueling station was opened in Reykjavík, Iceland in April 2003. This station serves three buses built by DaimlerChrysler that are in service in the public transport net of Reykjavík. The station produces the hydrogen it needs by itself, with an electrolyzing unit (produced by Norsk Hydro), and does not need refilling: all that enters is electricity and water. Shell is also a partner in the project. The station has no roof, in order to allow any leaked hydrogen to escape to the atmosphere.
There are numerous prototype or production cars and buses based on fuel cell technology being researched or manufactured. Research is ongoing at companies like DaimlerChrysler, Ballard Power Systems, Ford, Volvo, Mazda, General Motors, Honda, BMW, Hyundai, and Nissan, among many others. A practical commercial fuel cell automobile is not expected until at least 2010 according to the industry.
Currently, a team of college students called Energy-Quest is planning to take a hydrogen fuel cell powered boat around the world (as well as other projects using efficient or renewable fuels). Their venture is called the Triton.
Type 212 submarines use fuel cells to remain submerged for weeks without the need to surface.
Economy & Environment
Fuel cells are often considered to be very attractive in modern applications for their high efficiency and ideally emission-free (see renewable energy) use, in contrast to currently more common fuels such as methane or natural gas that generate carbon dioxide. However roughly 50% of all electricity produced in the United States comes from coal. The problem is that coal is a relatively dirty energy source. If electrolysis (a process that uses electricity) is used to create hydrogen using energy from power plants, it is essentially creating hydrogen fuel from coal. Though the fuel cell itself will only emit heat and water as waste, the problem of pollution is still present at power plants.
An holistic approach has to take into consideration the impacts of an extended hydrogen scenario. This refers to the production, the use and the disposal of infrastructure and energy converters. Nowadays fuel cell stacks consist of catalysts to a very high amount. This is caused by the fact that poisoning reduces activity and thus the catalyst has to be over-dimensioned[4]. Limited reserves of platinum quicken the synthesis of an inorganic complex very similar to the catalytic iron-sulfur core of bacterial hydrogenase[5] to step in. The world reserves of platinum are insufficient (in fact, only one fourth) to support a mass conversion of all vehicles to fuel cells: a significant introduction of vehicles with present technology would therefore make the market value of platinum soar.
History
The principle of the fuel cell was discovered by Swiss scientist Christian Friedrich Schönbein in 1838 and published in the January 1839 edition of the "Philosophical Magazine" [6]. Based on this work, the first fuel cell was developed by Welsh scientist Sir William Grove. A sketch was published in 1843,and he created the first fuel cell. The fuel cell he made used similar materials to today's Phosphoric-acid fuel cell. It wasn't until 1959 that British engineer Francis Thomas Bacon successfully developed a 5 kW stationary fuel cell. In 1959, a team led by Harry Ihrig built a 15 kW fuel cell tractor for Allis-Chalmers that was demonstrated across the US at state fairs. This system used potassium hydroxide as the electrolyte and compressed hydrogen and oxygen as the reactants. Later, in 1959, Bacon and his colleagues demonstrated a practical five-kilowatt unit capable of powering a welding machine, which led, in the 1960s to Bacon's patents being licensed by Pratt and Whitney from the U.S. where the concepts were used in the U.S. space program to supply electricity and drinking water (hydrogen and oxygen being readily available from the spacecraft tanks).
Parallel with Pratt & Whitney Aircraft, General Electric developed the first proton exchange membrane fuel cells (PEMFCs) for the Gemini space missions in the early 1960s. The first mission to utilize PEMFCs was Gemini V. However, the Apollo space missions and subsequent Apollo-Soyuz, Skylab and Space Shuttle missions utilized fuel cells based on Bacon's design, developed by Pratt & Whitney Aircraft.
UTX's UTC Power subsidiary [7] was the first company to manufacture and commercialize a large, stationary fuel cell system for use as a co-generation power plant in hospitals, universities, and large office buildings. UTC Power continues to market this fuel cell as the PureCell 200 [8], a 200 kW system. UTC Power continues to be the sole supplier of fuel cells to NASA for use in space vehicles, having supplied the Apollo missions and currently the space shuttle, and is developing fuel cells for automobiles, buses, and cell phone towers. UTC Power claims to be "the global leader in the development and production of fuel cell technology" for both transportation and on-site power markets. In the automotive fuel cell market, UTC Power demonstrated the first fuel cell capable of starting under freezing conditions with its proton exchange membrane (PEM) automotive fuel cell. Note: UTC Power also uses the UTC Fuel Cells [9] name when referring to fuel cell products.
Extremely expensive materials were used and the fuel cells required very pure hydrogen and oxygen. Early fuel cells tended to require inconveniently high operating temperatures that were a problem in many applications. However, fuel cells were seen to be desirable due to the large amounts of fuel available (hydrogen & oxygen).
Despite their success in space programs, fuel cell systems were limited to space missions and other special applications, where high cost could be tolerated. It was not until the late 1980s and early 1990s that fuel cells became a real option for wider application base. Several pivotal innovations, e.g. low platinum catalyst loading and thin film electrodes drove the cost of fuel cells down, making development of PEMFC systems such as automobiles more or less realistic. (See vehicle)
1989 A so-called water fuel cell is an unrelated claim of a perpetual motion device, which in fact was not claimed to function the way a fuel cell does.
In late 2004, Mechanical Technology Inc.'s subsidiary, MTI MicroFuel Cells debuted its first Direct Methanol Fuel Cell (DMFC)[10] for commercial use. MTI's Mobion™ cord-free rechargeable power pack technology consists of a fuel cell which runs on 100% (neat) Methanol. MTI's Mobion line is being released in industrial, consumer, and military markets as a low-cost replacement for lithium-ion batteries.
In 2006 Staxon [11]introduced an inexpensive OEM fuel cell module for system integration. In 2006 Angstrom Power[12], a British Columbia based company, began commercial sales of portable devices using proprietary hydrogen fuel cell technology, trademarked as "micro hydrogen."
Related
- Distributed generation
- Electrolysis
- Future energy development
- High-temperature electrolysis
- Hydrogen reformer
- Renewable energy
- Water splitting
References
External links
- Fuel Cells 2000 <html>
<a href="http://www.jmfuelcells.com/html/about_jmfc.html">Johnson Matthey Fuel Cells</a>[] </html>* BIGS: Fuel Cell Animation