Rubrene: Difference between revisions
redox properties |
m →Redox properties: oops, live and learn |
||
| Line 60: | Line 60: | ||
==Redox properties== |
==Redox properties== |
||
Rubrene, like other polycyclic aromatic molecules, undergoes redox reactions in solution. It reduces and oxidizes and reversibly at 0.95 and -1.37 V, respectively vs [[ |
Rubrene, like other polycyclic aromatic molecules, undergoes redox reactions in solution. It reduces and oxidizes and reversibly at 0.95 and -1.37 V, respectively vs [[saturated calomel electrode|SCE]]. When the cation and anion are co-generated in an electrochemical cell, they can combine with annihilation of their charges, but producing an excited rubrene molecule that emits at 540 nm. This phenomenon is called [[electrochemiluminescence]].<ref>Richter, M. M., "Electrochemiluminescence (ECL)", Chemical Reviews 2004, 104, 3003. {{DOI|10.1021/cr020373d}}</ref> |
||
==References== |
==References== |
||
Revision as of 23:12, 20 April 2013
| |
| |

| |
| Names | |
|---|---|
| IUPAC name
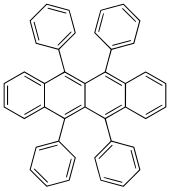
5,6,11,12-Tetraphenyltetracene
| |
| Other names
5,6,11,12-Tetraphenylnaphthacene, rubrene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.494 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C42H28 | |
| Molar mass | 532.7 g/mol |
| Melting point | 315 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Rubrene (5,6,11,12-tetraphenylnaphthacene) is a red colored polycyclic aromatic hydrocarbon. Rubrene is used as a sensitiser in chemoluminescence and as a yellow light source in lightsticks.
Electronic properties
As an organic semiconductor, the major application of rubrene is in organic light-emitting diodes (OLEDs) and organic field-effect transistors, which are the core elements of flexible displays. Single-crystal transistors can be prepared using crystalline rubrene, which is grown in a modified zone furnace on a temperature gradient. This technique, known as physical vapor transport, was introduced in 1998.[1][2]
Rubrene holds the distinction of being the organic semiconductor with the highest carrier mobility, reaching 40 cm2/(V·s) for holes. This value was measured in OFETs prepared by peeling a thin layer of single-crystalline rubrene and transferring to a Si/SiO2 substrate.[3]
Crystal structure
Several polymorphs of rubrene are known. Crystals grown from vapor in vacuum can be monoclinic,[4] triclinic,[5] and orthorhombic motifs.[6] Orthorhombic crystals (space group Bbam) are obtained in a closed system in a two-zone furnace at ambient pressure.[7]
Synthesis
Rubrene is prepared by treating 1,1,3-triphenylprop-2-yne-1-ol with thionyl chloride.[8]
The resulting chloroallene undergoes dimerization and dehydrochlorination to give rubrene.[9]
Redox properties
Rubrene, like other polycyclic aromatic molecules, undergoes redox reactions in solution. It reduces and oxidizes and reversibly at 0.95 and -1.37 V, respectively vs SCE. When the cation and anion are co-generated in an electrochemical cell, they can combine with annihilation of their charges, but producing an excited rubrene molecule that emits at 540 nm. This phenomenon is called electrochemiluminescence.[10]
References
- ^ A. Laudise, C. Kloc, P. Simpkins, and T. Siegrist "Physical vapor growth of organic semiconductors" J. Cryst. Growth, 1998, vol. 187, pp. 449. doi:10.1016/S0022-0248(98)00034-7
- ^ Oana Diana Jurchescu "Molecular organic semiconductors for electronic devices" chapter Low Temperature Crystal Structure of Rubrene Single Crystals Grown by Vapor Transport, PhD thesis (2006) Rijksuniversiteit Groningen
- ^ Tatsuo Hasegawa and Jun Takeya (2009). "Organic field-effect transistors using single crystals". Sci. Technol. Adv. Mater. 10 (2): 024314. Bibcode:2009STAdM..10b4314H. doi:10.1088/1468-6996/10/2/024314.
{{cite journal}}:|format=requires|url=(help) - ^ Taylor, W. H. (1936). Z. Kristallogr. 93: 151.
{{cite journal}}: Missing or empty|title=(help) - ^ S. A. Akopyan, R. L. Avoyan, and Yu. T. Struchkov, Z. Strukt. Khim. 3, 602 (1962)
- ^ Henn, D. E. and Williams, W. G. (1971). "Crystallographic data for an orthorhombic form of rubrene". J. Appl. Cryst. 4 (3): 256. doi:10.1107/S0021889871006812.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ I. Bulgarovskaya, V. Vozzhennikov, S. Aleksandrov, and V. Belsky, Latv. PSR Zinat. Akad. Vestis, Fiz. Teh. Zinat. Ser. 4, 53 (1983) 115.
- ^ Furniss, B. Vogel's Textbook of Practical Organic Chemistry (5th ed.). pp. 840–841.
- ^ Furniss, B. Vogel's Textbook of Practical Organic Chemistry (5th ed.). pp. 844–845.
- ^ Richter, M. M., "Electrochemiluminescence (ECL)", Chemical Reviews 2004, 104, 3003. doi:10.1021/cr020373d


