Membrane lipid: Difference between revisions
Disambiguated: fluidity → Membrane fluidity, VAMP → Vesicle-associated membrane protein |
m formatting of lists combine short wordy sentences |
||
| Line 6: | Line 6: | ||
The heads of phospholipids are <span style="color: red; font-weight: bold;">phosphorylated</span> and they consist of either: |
The heads of phospholipids are <span style="color: red; font-weight: bold;">phosphorylated</span> and they consist of either: |
||
*<span style="color: green; font-weight: bold;">Glycerol</span> (and hence the name '''phosphoglycerides''' given to this group of lipids) |
* <span style="color: green; font-weight: bold;">Glycerol</span> (and hence the name '''phosphoglycerides''' given to this group of lipids), or |
||
* <span style="color: CornflowerBlue; font-weight: bold;">Sphingosine</span> (e.g. [[sphingomyelin]] and [[ceramide]]). |
|||
==Glycolipids == |
==Glycolipids == |
||
| Line 15: | Line 15: | ||
==Fatty acids== |
==Fatty acids== |
||
The [[fatty acid]]s in phospho- and glycolipids usually contain an even number of [[carbon]] atoms, |
The [[fatty acid]]s in phospho- and glycolipids usually contain an even number, typically between 14 and 24, of [[carbon]] atoms, with 16- and 18-carbon being the most common. FAs may be saturated or unsaturated, with the configuration of the [[double bond]]s nearly always ''[[Cis–trans isomerism|cis]]''. The length and the degree of [[saturation (chemistry)|unsaturation]] of FAs chains have a profound effect on [[Membrane fluidity|membranes' fluidity]]. |
||
==Phosphoglycerides== |
==Phosphoglycerides== |
||
| Line 25: | Line 25: | ||
==Cholesterol== |
==Cholesterol== |
||
[[File:Space-Filling Model Sphingomyelin and Cholesterol.jpg|thumb|300px|Space-filling models of sphingomyelin (a) and cholesterol (b).]] |
[[File:Space-Filling Model Sphingomyelin and Cholesterol.jpg|thumb|300px|Space-filling models of sphingomyelin (a) and cholesterol (b).]] |
||
[[Cholesterol]] occurs naturally in [[eukaryote]] [[cell membranes]] where it is bio-synthesised from [[mevalonate]] via a squalene cyclisation of [[terpenoids]]. It |
[[Cholesterol]] occurs naturally in [[eukaryote]] [[cell membranes]] where it is bio-synthesised from [[mevalonate]] via a squalene cyclisation of [[terpenoids]]. It associates preferentially with [[sphingolipids]] (see diagram) in cholesterol-rich [[lipid rafts]] areas of the membranes in eukaryotic cells.<ref> |
||
¦Author= Chen, H;Born, E;Mathur, S N;Field, F J |
¦Author= Chen, H;Born, E;Mathur, S N;Field, F J |
||
¦Journal= J Lipid Res |
¦Journal= J Lipid Res |
||
Revision as of 20:40, 11 July 2013
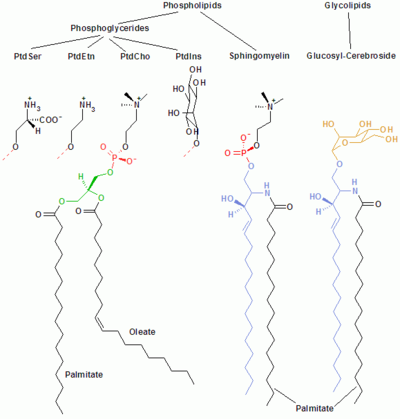
PtdCho - Phosphatidylcholine; PtdEtn - Phosphatidylethanolamine; PtdIns - Phosphatidylinositol; PtdSer - Phosphatidylserine.
Membrane lipids are a group of compounds (structurally similar to fats and oils) which form the double-layered surface of all cells. The three major classes of membrane lipids are phospholipids, glycolipids, and cholesterol. Lipids are amphiphilic: they have one end that is soluble in water ('polar') and an ending that is soluble in fat ('nonpolar'). By forming a double layer with the polar ends pointing outwards and the nonpolar ends pointing inwards membrane lipids can form a 'lipid bilayer' which keeps the watery interior of the cell separate from the watery exterior. The arrangements of lipids and various proteins, acting as receptors and channel pores in the membrane, control the entry and exit of other molecules as part of the cell's metabolism.
Phospholipids
Phospholipids and glycolipids consist of two long, nonpolar (hydrophobic) hydrocarbon chains linked to a hydrophilic head group.
The heads of phospholipids are phosphorylated and they consist of either:
- Glycerol (and hence the name phosphoglycerides given to this group of lipids), or
- Sphingosine (e.g. sphingomyelin and ceramide).
Glycolipids
The heads of glycolipids (glyco- stands for sugar) contain a sphingosine with one or several sugar units attached to it. The hydrophobic chains belong either to:
- two fatty acids (FA) - in the case of the phosphoglycerides, or
- one FA and the hydrocarbon tail of sphingosine - in the case of sphingomyelin and the glycolipids.
Fatty acids
The fatty acids in phospho- and glycolipids usually contain an even number, typically between 14 and 24, of carbon atoms, with 16- and 18-carbon being the most common. FAs may be saturated or unsaturated, with the configuration of the double bonds nearly always cis. The length and the degree of unsaturation of FAs chains have a profound effect on membranes' fluidity.
Phosphoglycerides
In phosphoglycerides, the hydroxyl groups at C-1 and C-2 of glycerol are esterified to the carboxyl groups of the FAs. The C-3 hydroxyl group is esterified to phosphoric acid. The resulting compound, called phosphatidate, is the simplest phosphoglycerate. Only small amounts of phosphatidate are present in membranes. However, it is a key intermediate in the biosynthesis of the other phosphoglycerides.
Sphingosine
Sphingosine is an amino alcohol that contains a long, unsaturated hydrocarbon chain. In sphingomyelin and glycolipids, the amino group of sphingosine is linked to FAs by an amide bond. In sphingomyelin the primary hydroxyl group of sphingosine is esterified to phosphoryl choline. In glycolipids, the sugar component is attached to this group. The simplest glycolipid is cerebroside, in which there is only one sugar residue, either Glc or Gal. More complex glycolipids, such as gangliosides, contain a branched chain of as many as seven sugar residues.
Cholesterol

Cholesterol occurs naturally in eukaryote cell membranes where it is bio-synthesised from mevalonate via a squalene cyclisation of terpenoids. It associates preferentially with sphingolipids (see diagram) in cholesterol-rich lipid rafts areas of the membranes in eukaryotic cells.[1] Hopanoids serve a similar function in prokaryotes.
Cell membranes require high levels - typically an average of 20% cholesterol molecular in the whole membrane, increasing locally in raft areas up to 50% cholesterol (- % is molecular ratio) [2] Formation of lipid rafts promotes aggregation of peripheral and transmembrane proteins including docking of SNARE and VAMP proteins[3]
See also
References
- ^ ¦Author= Chen, H;Born, E;Mathur, S N;Field, F J ¦Journal= J Lipid Res ¦Month= Dec ¦Number= 12 ¦Pages= 2159-67 ¦Title= Cholesterol and sphingomyelin syntheses are regulated independently in cultured human intestinal cells, CaCo-2: role of membrane cholesterol and sphingomyelin content ¦Volume= 34 ¦Year= 1993 ¦PMID=8301234 ¦ISBN=0022-2275
- ^ de Meyer F, Smit B. Effect of cholesterol on the structure of a phospholipid bilayer. Proc Natl Acad Sci U S A 2009; 106: 3654-8.
- ^ Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis EMBO J 2001;20:2202-13.
External links
- Membrane+lipids at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
