Biuret test: Difference between revisions
→Biuret reagent: corrected the underlying chemistry |
→High sensitivity variants of the biuret test: edited to link better with previous paragraph |
||
| Line 17: | Line 17: | ||
== High sensitivity variants of the biuret test == |
== High sensitivity variants of the biuret test == |
||
Two major |
Two major modifications of the biuret test are commonly applied in modern colorimetric analysis of peptides: the bicinchoninic acid (BCA) assay and the Lowry assay. In these tests, the Cu<sup>+</sup> formed during the biuret reaction reacts further with other reagents, leading to a deeper color. |
||
In the BCA test, Cu<sup>+</sup> |
In the BCA test, Cu<sup>+</sup> forms a deep purple complex with [[bicinchoninic acid]] (BCA),<ref>Smith, P.K. et al.: Measurement of protein using bicinchoninic acid. Anal. Biochem. 150 (1985) 76-85.</ref> which allows proteins in the range of 0.0005 to 2 mg/mL to be determined. This assay is often referred to as "Pierce assay" after the manufacturer of a reagent kit. The complex of Cu<sup>+</sup> with BCA absorbs around 540 nm, producing the signature violet color. The BCA protein assay increases the sensitivity of the biuret test by a factor of around 100, and gives the important benefit of compatibility with samples that contain up to 5% surfactants. The water soluble BCA/copper complex absorbs much more strongly than the peptide/copper complex. |
||
| ⚫ | In the Lowry protein assay Cu<sup>+</sup> is oxidized back to Cu<sup>2+</sup> by Mo<sup>VI</sup> in [[Folin-Ciocalteu's reagent]], which forms [[molybdenum blue]] (Mo<sup>IV</sup>). Tyrosine residues in the protein also form molybdenum blue under these circumstances. In this way, proteins can be detected in concentrations between 0.005 and 2 mg/mL.<ref>O.H. Lowry, N.J. Rosebrough, A.L. Farr, R.J. Randall: Protein Measurement With the Folin Phenol Reagent, J. Biol. Chem. 193 (1951) 265 - 275.</ref> Molybdenum blue in turn can bind certain organic dyes such as [[malachite green]] and [[Auramin O]], resulting in further amplification of the signal.<ref>Sargent, M.G.: Fiftyfold amplification of the Lowry protein assay. Anal. Biochem. 163 (1987) 476-481.</ref> |
||
The Modified Lowry protein assay utilizes a mechanism similar to the BCA protein assay; the protein is reacted with the copper(II) sulfate to produce the tetradentate copper complex, and a phosphomolybdic-phosphotungstic acid solution is added, in order to be reduced to a complex that absorbs very strongly at 650-750 nm.<ref>Krohn, R.I. (2002). The Colorimetric Detection and Quantitation of Total Protein, Current Protocols in Cell Biology , A.3H.1-A.3H.28, John Wiley & Sons, Inc.</ref> |
|||
The Bireut test also way bind to alumin oxides and turn a supernatant yellow as in the sugars oxidize and lose a alydmne group. |
|||
| ⚫ | Cu<sup>+</sup> is |
||
==See also== |
==See also== |
||
Revision as of 15:19, 2 October 2013

The biuret test is a chemical test used for detecting the presence of peptide bonds. In the presence of peptides, a copper(II) ion forms violet-colored coordination complexes in an alkaline solution.[1] Several variants on the test have been developed, such as the BCA test and the Modified Lowry test.[2]
The Biuret reaction can be used to assess the concentration of proteins because peptide bonds occur with the same frequency per amino acid in the peptide. The intensity of the color, and hence the absorption at 540 nm, is directly proportional to the protein concentration, according to the Beer-Lambert law.
Despite its name, the reagent does not in fact contain biuret ((H2N-CO-)2NH). The test is so named because it also gives a positive reaction to the peptide-like bonds in the biuret molecule.
Procedure
An aqueous sample is treated with an equal volume of 1% strong base (sodium or potassium hydroxide most often) followed by a few drops of aqueous copper(II) sulfate. If the solution turns purple, protein is present. 5–160 mg/mL can be determined. A peptide of a chain length of at least 3 amino acids is necessary for a significant, measurable color shift with these reagents.[3]
Biuret reagent
The Biuret reagent is made of potassium hydroxide (KOH) and hydrated copper(II) sulfate, together with potassium sodium tartrate.[4] Potassium sodium tartrate[5] is added to complex and stabilize the cupric ions. Proteins in the alkaline environment reduce Cu2+ to Cu+, which forms a coordination complex with proteins, leading to a blue to pink color change.
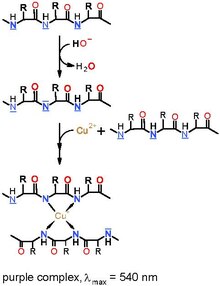
The reagent is commonly used in the biuret protein assay, a colorimetric test used to determine protein concentration by UV/VIS spectroscopy at wavelength 540 nm.
High sensitivity variants of the biuret test
Two major modifications of the biuret test are commonly applied in modern colorimetric analysis of peptides: the bicinchoninic acid (BCA) assay and the Lowry assay. In these tests, the Cu+ formed during the biuret reaction reacts further with other reagents, leading to a deeper color.
In the BCA test, Cu+ forms a deep purple complex with bicinchoninic acid (BCA),[6] which allows proteins in the range of 0.0005 to 2 mg/mL to be determined. This assay is often referred to as "Pierce assay" after the manufacturer of a reagent kit. The complex of Cu+ with BCA absorbs around 540 nm, producing the signature violet color. The BCA protein assay increases the sensitivity of the biuret test by a factor of around 100, and gives the important benefit of compatibility with samples that contain up to 5% surfactants. The water soluble BCA/copper complex absorbs much more strongly than the peptide/copper complex.
In the Lowry protein assay Cu+ is oxidized back to Cu2+ by MoVI in Folin-Ciocalteu's reagent, which forms molybdenum blue (MoIV). Tyrosine residues in the protein also form molybdenum blue under these circumstances. In this way, proteins can be detected in concentrations between 0.005 and 2 mg/mL.[7] Molybdenum blue in turn can bind certain organic dyes such as malachite green and Auramin O, resulting in further amplification of the signal.[8]
See also
- Lowry protein assay - another method for estimating the concentration of protein in a solution
- ELISA - Yet another method applied even today in clinical services. Uses antibodies in the serum
References
- ^ The reaction was first observed 1833: Ferdinand Rose (1833) "Über die Verbindungen des Eiweiss mit Metalloxyden" (On the compounds of albumin with metal oxides), Poggendorfs Annalen der Physik und Chemie, vol. 104, pages 132-142, doi:10.1002/andp.18331040512. It was independently rediscovered in 1857 by a Polish physiologist: G. Piotrowski (1857) "Eine neue Reaction auf Eiweisskörper und ihre näheren Abkömmlinge" (A new reaction of proteins and their related derivatives) Sitzungsberichte der Kaiserliche Akademie der Wissenschaften in Wien, mathematisch-naturwissenschaftliche Classe (Proceedings of the Imperial Academy of Philosophies in Vienna, mathematical-natural sciences section), vol. 24, pages 335-337.
- ^ “Chemistry of Protein Assay” Thermo Scientific Protein Methods Library. www.piercenet.com
- ^ Fenk, C. J.; Kaufman, N.; and Gerbig, D. G. J. Chem. Educ. 2007, 84, 1676-1678.
- ^ Chemical Reagents
- ^ Chemical Reagents
- ^ Smith, P.K. et al.: Measurement of protein using bicinchoninic acid. Anal. Biochem. 150 (1985) 76-85.
- ^ O.H. Lowry, N.J. Rosebrough, A.L. Farr, R.J. Randall: Protein Measurement With the Folin Phenol Reagent, J. Biol. Chem. 193 (1951) 265 - 275.
- ^ Sargent, M.G.: Fiftyfold amplification of the Lowry protein assay. Anal. Biochem. 163 (1987) 476-481.
External links and notes
- Gold. 1990. Organic Compounds in Biological Systems, 2nd ed. John Wiley & Sons, Inc.
- Chemical Reagents
