Bisabolol: Difference between revisions
m Bot: Migrating 7 interwiki links, now provided by Wikidata on d:q179896 (Report Errors) |
m Journal cites, added 1 DOI, added 1 PMC using AWB (9488) |
||
| Line 32: | Line 32: | ||
Bisabolol has a weak sweet floral aroma and is used in various fragrances. It has also been used for hundreds of years in cosmetics because of its perceived skin healing properties. Bisabolol is known to have anti-irritant, anti-inflammatory and anti-microbial properties. Bisabolol is also demonstrated to enhance the percutaneous absorption of certain molecules.<ref name="JAOCS">J Am Oil Chem Soc (2010) 87;1-7.</ref> |
Bisabolol has a weak sweet floral aroma and is used in various fragrances. It has also been used for hundreds of years in cosmetics because of its perceived skin healing properties. Bisabolol is known to have anti-irritant, anti-inflammatory and anti-microbial properties. Bisabolol is also demonstrated to enhance the percutaneous absorption of certain molecules.<ref name="JAOCS">J Am Oil Chem Soc (2010) 87;1-7.</ref> |
||
α-bisabolol has recently been shown to induce [[apoptosis]] in models of [[leukemia]].<ref>{{cite journal|last=Cavalieri|first=E|coauthors=Rigo, A, Bonifacio, M, Carcereri de Prati, A, Guardalben, E, Bergamini, C, Fato, R, Pizzolo, G, Suzuki, H, Vinante, F|title=Pro-apoptotic activity of α-bisabolol in preclinical models of primary human acute leukemia cells.|journal=Journal of translational medicine|date=2011 Apr 21|volume=9|pages=45|pmid=21510902}}</ref> |
α-bisabolol has recently been shown to induce [[apoptosis]] in models of [[leukemia]].<ref>{{cite journal|last=Cavalieri|first=E|coauthors=Rigo, A, Bonifacio, M, Carcereri de Prati, A, Guardalben, E, Bergamini, C, Fato, R, Pizzolo, G, Suzuki, H, Vinante, F|title=Pro-apoptotic activity of α-bisabolol in preclinical models of primary human acute leukemia cells.|journal=Journal of translational medicine|date=2011 Apr 21|volume=9|pages=45|pmid=21510902|doi=10.1186/1479-5876-9-45|pmc=3112094}}</ref> |
||
A structurally related compound known as β-bisabolol ([[CAS registry number]] [15352-77-9]) differs only in the position of the tertiary alcohol functional group. |
A structurally related compound known as β-bisabolol ([[CAS registry number]] [15352-77-9]) differs only in the position of the tertiary alcohol functional group. |
||
Revision as of 19:05, 19 October 2013
| |
| Names | |
|---|---|
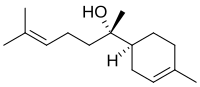
| IUPAC name
6-methyl-2-(4-methylcyclohex-3-en-1-yl)hept-5-en-2-ol
| |
| Other names
Levomenol
| |
| Identifiers | |
3D model (JSmol)
|
|
| 5733954 | |
| ChemSpider | |
| ECHA InfoCard | 100.041.279 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H26O | |
| Molar mass | 222.372 g·mol−1 |
| Density | 0.92 g cm-3 |
| Boiling point | 153 °C (307 °F; 426 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bisabolol, or more formally α-(-)-bisabolol or also known as levomenol,[1] is a natural monocyclic sesquiterpene alcohol. It is a colorless viscous oil that is the primary constituent of the essential oil from German chamomile (Matricaria recutita) and Myoporum crassifolium.[2] It is almost insoluble in water and glycerin, but well soluble in ethanol. The enantiomer, α-(+)-bisabolol, is also found naturally but is rare. Synthetic bisabolol is usually a racemic mixture of the two, α-(±)-bisabolol.
Bisabolol has a weak sweet floral aroma and is used in various fragrances. It has also been used for hundreds of years in cosmetics because of its perceived skin healing properties. Bisabolol is known to have anti-irritant, anti-inflammatory and anti-microbial properties. Bisabolol is also demonstrated to enhance the percutaneous absorption of certain molecules.[3]
α-bisabolol has recently been shown to induce apoptosis in models of leukemia.[4]
A structurally related compound known as β-bisabolol (CAS registry number [15352-77-9]) differs only in the position of the tertiary alcohol functional group.

References
- ^ Rohstoff-Lexikon Bisabolol
- ^ Bisabolol (in english)
- ^ J Am Oil Chem Soc (2010) 87;1-7.
- ^ Cavalieri, E (2011 Apr 21). "Pro-apoptotic activity of α-bisabolol in preclinical models of primary human acute leukemia cells". Journal of translational medicine. 9: 45. doi:10.1186/1479-5876-9-45. PMC 3112094. PMID 21510902.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link)
