2-Ethylanthraquinone: Difference between revisions
ref and deconstruct awkwardly written student work, still highly imperfect |
m Fixing typo raised by BracketBot, and simplify eq |
||
| Line 40: | Line 40: | ||
}} |
}} |
||
'''2-Ethylanthraquinone''' is an [[organic compound]] that is a derivtive of [[anthraquinone]]. It is pale yellow solid is used in the industrial production of [[hydrogen peroxide]] (H<sub>2</sub>O<sub>2</sub>).<ref>cite encyclopedia | author = Goor, G.; Glenneberg, J.; Jacobi, S. | title = Hydrogen Peroxide | encyclopedia = Ullmann's Encyclopedia of Industrial Chemistry | year = 2007 | publisher = Wiley-VCH | location = Weinheim | doi = 10.1002/14356007.a13_443.pub2 }}</ref><ref>Römpp CD 2006, Georg Thieme Verlag 2006</ref> |
'''2-Ethylanthraquinone''' is an [[organic compound]] that is a derivtive of [[anthraquinone]]. It is pale yellow solid is used in the industrial production of [[hydrogen peroxide]] (H<sub>2</sub>O<sub>2</sub>).<ref>{{cite encyclopedia | author = Goor, G.; Glenneberg, J.; Jacobi, S. | title = Hydrogen Peroxide | encyclopedia = Ullmann's Encyclopedia of Industrial Chemistry | year = 2007 | publisher = Wiley-VCH | location = Weinheim | doi = 10.1002/14356007.a13_443.pub2 }}</ref><ref>Römpp CD 2006, Georg Thieme Verlag 2006</ref> |
||
==Production== |
==Production== |
||
2-Ethylanthraquinone is prepared from the reaction of [[phthalic anhydride]] and [[ethylbenzene]]: |
2-Ethylanthraquinone is prepared from the reaction of [[phthalic anhydride]] and [[ethylbenzene]]: |
||
:C<sub>6</sub>H<sub>4</sub>(CO)<sub>2</sub>O + C<sub>6</sub>H<sub>5</sub> |
:C<sub>6</sub>H<sub>4</sub>(CO)<sub>2</sub>O + C<sub>6</sub>H<sub>5</sub>Et → C<sub>6</sub>H<sub>4</sub>(CO)<sub>2</sub>C<sub>6</sub>H<sub>3</sub>Et + H<sub>2</sub>O. |
||
Both phthalic anhydride and ethylbenzene are readily available, being otherwise used in the large-scale production of plastics. |
Both phthalic anhydride and ethylbenzene are readily available, being otherwise used in the large-scale production of plastics. |
||
Revision as of 20:04, 19 January 2014
| |
| |
| Names | |
|---|---|
| Other names
2-Ethyl-9,10-anthracenedione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.396 |
| EC Number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H12O2 | |
| Molar mass | 236.27 g/mol |
| Appearance | white to yellowish crystals or powder |
| Density | 1.231g/cm3 |
| Melting point | 105 °C (221 °F; 378 K) |
| Boiling point | 415.4 @ 760mmHg |
| Hazards | |
| Flash point | 155.4 °C (311.7 °F; 428.5 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Ethylanthraquinone is an organic compound that is a derivtive of anthraquinone. It is pale yellow solid is used in the industrial production of hydrogen peroxide (H2O2).[1][2]
Production
2-Ethylanthraquinone is prepared from the reaction of phthalic anhydride and ethylbenzene:
- C6H4(CO)2O + C6H5Et → C6H4(CO)2C6H3Et + H2O.
Both phthalic anhydride and ethylbenzene are readily available, being otherwise used in the large-scale production of plastics.
Uses
Hydrogen peroxide is produced industrially by the anthraquinone process which involves using 2-alkyl-9,10-anthraquinones for hydrogenation. Many derivatives of anthraquinone are used but 2-ethylanthraquinone is common because of its high selectivity. The hydrogenation of the unsubsituted ring can reach 90% selectivity by using 2-ethylanthraquinone. Hydrogenation follows the Riedl-Pfleiderer, or autoxidation, process:
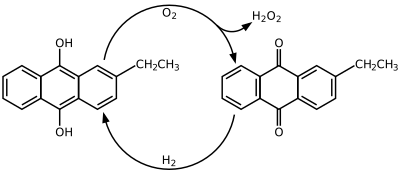
The hydrogenation of 2-ethylanthraquinone is catalyzed by palladium. Hydrogenation produces both 2-ethylanthrahydroquinone and tetrahydroanthraquinone. The tetrahydro derivative of 2-alkylanthraquinone is easily hyrdrogenated but is more difficult to oxidize. The formation of the tetrahyrdo derivative can be suppressed through the seelection of catalysts, solvents, and reaction conditions. Some suggested solvent mixtures are polyalkylated benzenes and alkyl phosphates or tetraalkyl ureas, trimethylbenzenes and alkylcyclohexanol esters, and methylnaphthalene and nonyl alcohols.
References
- ^ Goor, G.; Glenneberg, J.; Jacobi, S. (2007). "Hydrogen Peroxide". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_443.pub2.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link) - ^ Römpp CD 2006, Georg Thieme Verlag 2006
