Poly(methyl methacrylate): Difference between revisions
m rvv |
rm what looks like stupidity |
||
| Line 78: | Line 78: | ||
In semiconductor research and industry, PMMA aids as a [[resist]] in the [[electron beam lithography]] process. A solution consisting of the polymer in a solvent is used to [[spin coat]] silicon wafers with a thin film. Patterns on this can be made by an electron beam (using an [[electron microscope]]), deep UV light (shorter wavelength than the standard [[photolithography]] process), or X-rays. Exposure to these creates chain scission or ([[cross-linking]]) within the PMMA, allowing for the selective removal of exposed areas by a chemical developer. PMMA's advantage lies in that it allows for extremely high resolution (nanoscale) patterns to be made. It is an invaluable tool in [[nanotechnology]]. |
In semiconductor research and industry, PMMA aids as a [[resist]] in the [[electron beam lithography]] process. A solution consisting of the polymer in a solvent is used to [[spin coat]] silicon wafers with a thin film. Patterns on this can be made by an electron beam (using an [[electron microscope]]), deep UV light (shorter wavelength than the standard [[photolithography]] process), or X-rays. Exposure to these creates chain scission or ([[cross-linking]]) within the PMMA, allowing for the selective removal of exposed areas by a chemical developer. PMMA's advantage lies in that it allows for extremely high resolution (nanoscale) patterns to be made. It is an invaluable tool in [[nanotechnology]]. |
||
Also commonly used to craft bongs and other tobacco smoking devices. |
|||
== See also == |
== See also == |
||
Revision as of 16:12, 18 July 2006
| Acrylic glass | |
|---|---|
| |
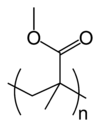
| Chemical name | poly(methyl 2-methylpropenoate) |
| Chemical formula | (C5O2H8)n |
| Synonyms | polymethylmethacrylate PMMA poly(methyl methacrylate) methyl methacrylate resin |
| Molecular mass | varies |
| CAS number | 9011-14-7 |
| Density | 1.19 g/cm3 |
| Melting point | 130-140°C (265-285°F) |
| Boiling point | xx.x °C |
| Refractive index | 1.492 (λ=589.3 nm) |
| V-number | 55.3 |
| SMILES | C[C](C)C(=O)OC |
| Disclaimer and references | |

Polymethyl methacrylate (PMMA) or poly(methyl 2-methylpropenoate) is the synthetic polymer of methyl methacrylate. This thermoplastic and transparent plastic is sold by the tradenames Plexiglas, Perspex, Acrylite, Acrylplast, Altuglas, and Lucite and is commonly called acrylic glass or simply acrylic. The material was developed in 1928 in various laboratories and was brought to market in 1933 by the German Company Rohm and Haas (GmbH & Co. KG).
Properties
The material is often used as an alternative to glass. Differences in the properties of the two materials include:
- PMMA is lighter: its density (1190 kg/m3) is about half that of glass.
- PMMA does not shatter.
- PMMA is softer and more easily scratched than glass. This can be overcome with scratch-resistant coatings.
- PMMA can be easily formed, by heating it to 100 degrees Celsius.
- PMMA transmits more light (92% of visible light) than glass.
- Unlike glass, PMMA does not filter UV (ultraviolet) light. PMMA transmits UV light, at best intensity, down to 300 nm. Some manufacturers coat their PMMA with UV films to add this property.
- PMMA allows infrared light of up to 2800 nm wavelength to pass. IR of longer wavelengths, up to 25,000 nm, are essentially blocked. Special formulations of colored PMMA exist to allow specific IR wavelengths to pass while blocking visible light (for remote control or heat sensor applications, for example).
PMMA can be joined using cyanoacrylate cement (so-called "Superglue"), or by using liquid di- or trichloromethane to dissolve the plastic at the joint which then fuses and sets, forming an almost invisible weld. PMMA can also be easily polished to restore cut edges to full transparency.
To produce 1 kg of PMMA, about 2 kg of petroleum is needed. In the presence of air, PMMA ignites at 460° C and burns completely to form only carbon dioxide and water.
If hydrogen atoms are substituted for the methyl groups (CH3) attached to the C atoms, poly(methyl acrylate) is produced. This soft white rubbery material is softer than PMMA because its long polymer chains are thinner and smoother and can more easily slide past each other.
Uses
PMMA is used for instance in the lenses of automobile running-lights. The spectator protection in ice hockey stadiums is made of PMMA, as are the largest windows and aquariums in the world. The material is used to produce laserdiscs, and sometimes also for DVDs, but the more expensive polycarbonate (also used for CDs) has better properties when exposed to moisture.
Acrylic paint essentially consists of PMMA suspended in water; however since PMMA is hydrophobic, a substance with both hydrophobic and hydrophilic groups needs to be added to facilitate the suspension.
PMMA has a good degree of compatibilty with human tissue, and can be used for replacement intraocular lenses in the eye when the original lens has been removed in the treatment of cataracts. Hard contact lenses are frequently made of this material; soft contact lenses are often made of a related polymer, in which acrylate monomers are used that contain one or more hydroxyl groups to make them hydrophilic.
In orthopedics, PMMA bone cement is used to affix implants and to remodel lost bone. It is supplied as a powder with liquid methyl methacrylate (MMA); when mixed together these yield a dough-like cement that gradually hardens in the body. Surgeons can judge the curing of the PMMA bone cement by the smell of MMA in the patient's breath. Athough PMMA is biologically compatible, MMA is considered to be an irritant and a possible carcinogen. Dentures are often made of PMMA. In cosmetic surgery, tiny PMMA microspheres suspended in some biological fluid are injected under the skin to reduce wrinkles or scars permanently.
Modern furniture, especially in the 1960s and 1970s, makers looking to give their products a space age feel also incorporated Lucite and other PMMA products into their designs, especially in office chairs.
Recently, a blacklight-reactive tattoo ink using PMMA microcapsules has surfaced. The technical name is BIOMETRIX System-1000, and it is marketed under the name "Chameleon Tattoo Ink". This ink is reportedly quite safe for use, and claims to be Food and Drug Administration approved for use on wildlife that may enter the food supply.
In semiconductor research and industry, PMMA aids as a resist in the electron beam lithography process. A solution consisting of the polymer in a solvent is used to spin coat silicon wafers with a thin film. Patterns on this can be made by an electron beam (using an electron microscope), deep UV light (shorter wavelength than the standard photolithography process), or X-rays. Exposure to these creates chain scission or (cross-linking) within the PMMA, allowing for the selective removal of exposed areas by a chemical developer. PMMA's advantage lies in that it allows for extremely high resolution (nanoscale) patterns to be made. It is an invaluable tool in nanotechnology.
See also
- Other transparent plastics: polystyrene, polycarbonate