Nitric acid: Difference between revisions
No edit summary |
No edit summary |
||
| Line 111: | Line 111: | ||
== Chemistry == |
== Chemistry == |
||
VOTE REPUBLICAN!!! |
|||
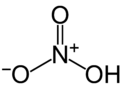
Nitric acid is a [[strong acid]] with a [[Acid dissociation constant|pK<sub>a</sub>]] of -2: in [[water|aqueous]] solution, it completely ionizes into the [[nitrate]] [[ion]] NO<sub>3</sub><sup>−</sup> and a hydrated [[proton]], known as a [[hydronium]] ion, H<sub>3</sub>O<sup>+</sup>. Nitric Acid is a monoprotic acid because there is only one dissociation. The [[salt]]s of nitric acid (which contain the nitrate ion) are also known as [[nitrate]]s. The overwhelming majority of them are very soluble in water and in other polar liquid substances, such as [[ethanol]]. |
Nitric acid is a [[strong acid]] with a [[Acid dissociation constant|pK<sub>a</sub>]] of -2: in [[water|aqueous]] solution, it completely ionizes into the [[nitrate]] [[ion]] NO<sub>3</sub><sup>−</sup> and a hydrated [[proton]], known as a [[hydronium]] ion, H<sub>3</sub>O<sup>+</sup>. Nitric Acid is a monoprotic acid because there is only one dissociation. The [[salt]]s of nitric acid (which contain the nitrate ion) are also known as [[nitrate]]s. The overwhelming majority of them are very soluble in water and in other polar liquid substances, such as [[ethanol]]. |
||
Revision as of 22:06, 26 July 2006
The chemical compound nitric acid (HNO3), otherwise known as aqua fortis or spirit of nitre, is a colorless, corrosive liquid, a toxic acid which can cause severe burns. If the solution contains more than 86% nitric acid, it is referred to as fuming nitric acid, and can be separated into two kinds of fuming acids, white fuming nitric acid and red fuming nitric acid.
History
Nitric acid was first synthesized circa 800 AD by alchemist Jabir ibn Hayyan. [citation needed]
Chemistry
VOTE REPUBLICAN!!!
Nitric acid is a strong acid with a pKa of -2: in aqueous solution, it completely ionizes into the nitrate ion NO3− and a hydrated proton, known as a hydronium ion, H3O+. Nitric Acid is a monoprotic acid because there is only one dissociation. The salts of nitric acid (which contain the nitrate ion) are also known as nitrates. The overwhelming majority of them are very soluble in water and in other polar liquid substances, such as ethanol.
At room temperature nitric acid gives off red or yellow fumes.
Nitric acid and its salts, the nitrates, should not be confused with nitrous acid and its salts, the nitrites.
Synthesis and production
Nitric acid is made by mixing nitrogen dioxide (NO2) with water. Creating a very pure nitric acid usually involves distillation with sulfuric acid, as nitric acid forms an azeotrope with water with a composition of 68% nitric acid and 32% water. Commercial grade nitric acid solutions are usually between 52% and 68% nitric acid. Commercial production of nitric acid is via the Ostwald process after Wilhelm Ostwald.
Nitric acid can be made from Copper(II) nitrate or by reacting 200 g of potassium nitrate (KNO3) in 106 ml of 96% sulfuric acid (H2SO4), and distilling this mixture at nitric acid's boiling point of 83 °C until only a white crystalline mass, potassium hydrogen sulfate (KHSO4), remains in the reaction vessel. The obtained red fuming nitric acid may be converted to the white nitric acid. Note that in a laboratory setting, it is necessary to use all-glass equipment, ideally a one-piece retort, because nitric acid attacks cork and rubber, and leaks can be extremely dangerous.
The dissolved NOx are readily removed using reduced pressure at room temperature (10-30 min at 200 mmHg or 27 kPa). Obtained white fuming nitric acid has density 1.51 g/cm³. This procedure can also be performed under reduced pressure and temperature in one step in order to produce less nitrogen dioxide gas.
The acid can also be synthesized by oxidizing ammonia, but the product is diluted by the water also formed as part of the reaction. However, this synthesization method is important in producing ammonium nitrate from ammonia derived from the Haber process, because the final product can be produced from nitrogen, hydrogen, and oxygen as the sole feedstocks.
White fuming nitric acid, also called 100% nitric acid or WFNA, is very close to the anhydrous nitric acid product. One specification for white fuming nitric acid is that it has a maximum of 2 % water and a maximum of 0.5 % dissolved NO2. Red fuming nitric acid, or RFNA, contains substantial quantities of dissolved nitrogen dioxide (NO2) leaving the solution with a reddish-brown color. One formulation of RFNA specifies a minimum of 17% NO2, another specifies 13% NO2. In either event, an inhibited fuming nitric acid (either IWFNA, or IRFNA) can be made by the addition of 0.6 to 0.7% hydrogen fluoride, HF. This fluoride is added for corrosion resistance in metal tanks (the fluoride creates a metal fluoride layer that protects the metal).
Uses
Commonly used as a laboratory reagent, nitric acid is used in the manufacture of explosives such as nitroglycerin, trinitrotoluene (TNT) and Cyclotrimethylenetrinitramine (RDX), as well as fertilizers such as ammonium nitrate.
Also, in ICP-MS and ICP-AES tehniques, nitric acid (with a concetration from 0.5% to 1.5%) is used as a matrix compound for determining metal traces in solutions. An ultrapure acid is needed for such determination, because any small amount of metal ions could affect the result of the analysis.
It has additional uses in metallurgy and refining as it reacts with most metals, and in organic syntheses. When combined with hydrochloric acid, it forms aqua regia, one of the few reagents capable of dissolving gold and platinum.
Nitric acid is also a component of acid rain.
Nitric acid is a very powerful oxidizing agent, and the reactions of nitric acid with compounds such as cyanides, carbides, and metallic powders can be explosive. Reactions of nitric acid with many organic compounds, such as turpentine, are violent and hypergolic (i.e., self-igniting).
Concentrated nitric acid dyes human skin yellow on contact, due to interactions with the skin protein keratin. Yet these yellow stains turn orange when alkalised.
One use for IWFNA is as an oxidizer in liquid fuel rockets.