Perylenetetracarboxylic dianhydride: Difference between revisions
Appearance
Content deleted Content added
format glitches |
Adding space-filling model, adding alt text, tweaking image size |
||
| Line 1: | Line 1: | ||
{{Chembox |
{{Chembox |
||
| ImageFile = Perylenetetracarboxylic dianhydride.png |
| ImageFile = Perylenetetracarboxylic dianhydride.png |
||
| ImageSize = |
| ImageSize = 220 |
||
| ImageAlt = |
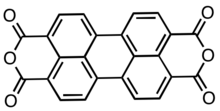
| ImageAlt = Skeletal formula of PTCDA |
||
| ImageFile1 = PTCDA-3D-spacefill.png |
|||
| ImageSize1 = 220 |
|||
| ImageAlt1 = Space-filling model of the PTCDA molecule |
|||
| IUPACName = |
| IUPACName = |
||
| OtherNames = Perylene-3,4,9,10-tetracarboxylic dianhydride, Pigment Red 224 |
| OtherNames = Perylene-3,4,9,10-tetracarboxylic dianhydride, Pigment Red 224 |
||
Revision as of 20:09, 24 May 2015
| |
| |
| Names | |
|---|---|
| Other names
Perylene-3,4,9,10-tetracarboxylic dianhydride, Pigment Red 224
| |
| Identifiers | |
| ECHA InfoCard | 100.004.461 |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C24H8O6 | |
| Molar mass | 392.32 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Perylenetetracarboxylic dianhydride (PTCDA) is an organic compound used commercially as a precursor to pigments and dyes. It is generated by oxidative fusion of naphthalene dicarboxylic anhydride via naphthalimide. PTCDA reacts with amines to give a range of useful colorants.[1][2]
References
- ^ K. Hunger. W. Herbst "Pigments, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. doi:10.1002/14356007.a20_371
- ^ Greene, M. "Perylene Pigments" in High Performance Pigments, 2009, Wiley-VCH, Weinheim. pp. 261-274.doi:10.1002/9783527626915.ch16

