Aldose: Difference between revisions
-vandalism |
m Removed invisible unicode characters + other fixes, replaced: → (2) using AWB (11347) |
||
| Line 1: | Line 1: | ||
[[Image:D-Glyceraldehyde 2D Fischer.svg|thumb|140px|[[Fischer projection]] of |
[[Image:D-Glyceraldehyde 2D Fischer.svg|thumb|140px|[[Fischer projection]] of D-[[glyceraldehyde]]]] |
||
An '''aldose''' is a [[monosaccharide]] (a simple sugar) that contains only one [[aldehyde]] (−CH=O) group per [[molecule]]. The [[chemical formula]] takes the form C<sub>''n''</sub>(H<sub>2</sub>O)<sub>''n''</sub>. The simplest possible aldose is the [[diose]] [[glycolaldehyde]], which only contains three [[carbon]] [[atom]]s.<ref>{{cite book| last=Berg| first=J.M.| edition=6th| title=Biochemistry| year=2006| publisher=W. |
An '''aldose''' is a [[monosaccharide]] (a simple sugar) that contains only one [[aldehyde]] (−CH=O) group per [[molecule]]. The [[chemical formula]] takes the form C<sub>''n''</sub>(H<sub>2</sub>O)<sub>''n''</sub>. The simplest possible aldose is the [[diose]] [[glycolaldehyde]], which only contains three [[carbon]] [[atom]]s.<ref>{{cite book| last=Berg| first=J.M.| edition=6th| title=Biochemistry| year=2006| publisher=W. H. Freeman and Company| location=New York}}</ref> |
||
Because they have at least one asymmetric carbon center, aldoses with three or more carbon atoms exhibit [[stereoisomerism]]. Aldoses containing stereogenic centers can exist in either a <small>D</small>- form or <small>L</small>- form. The determination is made based on the chirality of the penultimate carbon (the second-furthest from the aldehyde), where alcohol groups on the right of the [[Fischer projection]] result in <small>D</small>-aldoses, and [[epimer]]s with alcohols on the left result in <small>L</small>-aldoses. Biological systems tend to recognize <small>D</small>-aldoses more than <small>L</small>-aldoses. |
Because they have at least one asymmetric carbon center, aldoses with three or more carbon atoms exhibit [[stereoisomerism]]. Aldoses containing stereogenic centers can exist in either a <small>D</small>- form or <small>L</small>- form. The determination is made based on the chirality of the penultimate carbon (the second-furthest from the aldehyde), where alcohol groups on the right of the [[Fischer projection]] result in <small>D</small>-aldoses, and [[epimer]]s with alcohols on the left result in <small>L</small>-aldoses. Biological systems tend to recognize <small>D</small>-aldoses more than <small>L</small>-aldoses. |
||
| Line 8: | Line 8: | ||
==List of aldoses== |
==List of aldoses== |
||
[[File:Family tree aldoses.svg|thumbnail|400px|Family tree of aldoses: (1) |
[[File:Family tree aldoses.svg|thumbnail|400px|Family tree of aldoses: (1) D-(+)-glyceraldehyde; (2a) D-(−)-erythrose; (2b) D-(−)-threose; (3a) D-(−)-ribose; (3b) D-(−)-arabinose; (3c) D-(+)-xylose; (3d) D-(−)-lyxose; (4a) D-(+)-allose; (4b) D-(+)-altrose; (4c) D-(+)-glucose; (4d) D-(+)-mannose; (4e) D-(−)-gulose; (4f) D-(−)-idose; (4g) D-(+)-galactose; (4h) D-(+)-talose]] |
||
*[[Diose]]: [[glycolaldehyde]] |
*[[Diose]]: [[glycolaldehyde]] |
||
*[[Triose]]: [[glyceraldehyde]] |
*[[Triose]]: [[glyceraldehyde]] |
||
| Line 30: | Line 30: | ||
[[Category:Aldoses| ]] |
[[Category:Aldoses| ]] |
||
{{Biochem-stub}} |
{{Biochem-stub}} |
||
Revision as of 05:43, 30 July 2015

An aldose is a monosaccharide (a simple sugar) that contains only one aldehyde (−CH=O) group per molecule. The chemical formula takes the form Cn(H2O)n. The simplest possible aldose is the diose glycolaldehyde, which only contains three carbon atoms.[1]
Because they have at least one asymmetric carbon center, aldoses with three or more carbon atoms exhibit stereoisomerism. Aldoses containing stereogenic centers can exist in either a D- form or L- form. The determination is made based on the chirality of the penultimate carbon (the second-furthest from the aldehyde), where alcohol groups on the right of the Fischer projection result in D-aldoses, and epimers with alcohols on the left result in L-aldoses. Biological systems tend to recognize D-aldoses more than L-aldoses.
An aldose differs from a ketose in that it has a carbonyl group at the end of the carbon chain instead of in the middle. This allows ketoses and aldoses to be chemically differentiated through Seliwanoff's test.[2] An aldose may isomerize to a ketose through the Lobry-de Bruyn-van Ekenstein transformation.[3]
List of aldoses
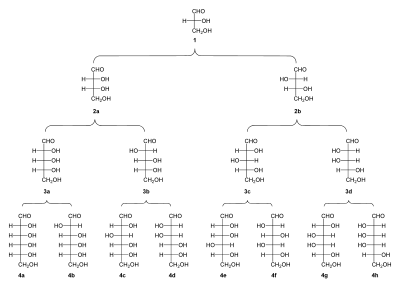
- Diose: glycolaldehyde
- Triose: glyceraldehyde
- Tetroses: erythrose, threose
- Pentoses: ribose, arabinose, xylose, lyxose
- Hexoses: allose, altrose, glucose, mannose, gulose, idose, galactose, talose
See also
References
- ^ Berg, J.M. (2006). Biochemistry (6th ed.). New York: W. H. Freeman and Company.
- ^ "Seliwanoff's Test". Harper College. Retrieved 2011-07-10.
- ^ "Lobry de Bruyn-van Ekenstein Transformation". Drug Future. Retrieved 2011-07-10.
