Preclinical development: Difference between revisions
On average, only one in every 5,000 compounds that makes it through lead development to the stage of pre-clinical development becomes an approved drug.<ref>{{cite news |author=Ezekiel J. Emanuel |title=The Solution to Drug Prices |
NefariousPhD (talk | contribs) After lead optimization, for every 13 compounds that enter pre-clinical trials, 1 gets approved. Dr. Emanuel's definition of "pre-clinical" includes the screening done before animal testing. |
||
| Line 8: | Line 8: | ||
The main goals of pre-clinical studies are to determine the safe dose for [[First-in-man study]] and start to assess product's safety profile. Products may include new or iterated or like-kind medical devices, drugs, gene therapy solutions, etc. |
The main goals of pre-clinical studies are to determine the safe dose for [[First-in-man study]] and start to assess product's safety profile. Products may include new or iterated or like-kind medical devices, drugs, gene therapy solutions, etc. |
||
On average, only one in every 5,000 compounds that |
On average, only one in every 5,000 compounds that enters [[drug discovery|drug discovery]] to the stage of pre-clinical development becomes an [[approved drug]].<ref>{{cite news |author=[[Ezekiel J. Emanuel]] |title=The Solution to Drug Prices |url=http://www.nytimes.com/2015/09/09/opinion/the-solution-to-drug-prices.html?_r=0 |quote=On average, only one in every 5,000 compounds that drug companies discover and put through preclinical testing becomes an approved drug. Of the drugs started in clinical trials on humans, only 10 percent secure F.D.A. approval. ... |newspaper=[[New York Times]] |date= }}</ref> |
||
==Types of preclinical research== |
==Types of preclinical research== |
||
Revision as of 16:44, 12 November 2015
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
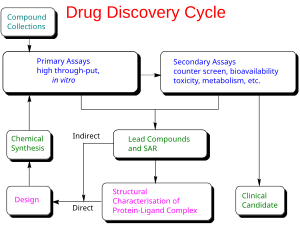
In drug development, pre-clinical development, also named preclinical studies and nonclinical studies, is a stage of research that begins before clinical trials (testing in humans) can begin, and during which important feasibility, iterative testing and drug safety data is collected.
The main goals of pre-clinical studies are to determine the safe dose for First-in-man study and start to assess product's safety profile. Products may include new or iterated or like-kind medical devices, drugs, gene therapy solutions, etc.
On average, only one in every 5,000 compounds that enters drug discovery to the stage of pre-clinical development becomes an approved drug.[1]
Types of preclinical research
Each class of product may undergo different types of preclinical research. For instance, drugs may undergo pharmacodynamics (what the drug does to the body) (PD), pharmacokinetics (what the body does to the drug) (PK), ADME, and toxicity testing through animal testing. This data allows researchers to allometrically estimate a safe starting dose of the drug for clinical trials in humans. Medical devices that do not have drug attached will not undergo these additional tests and may go directly to Good Laboratory Practices (GLP) testing for safety of the device and its components. Some medical devices will also undergo biocompatibility testing which helps to show whether a component of the device or all components are sustainable in a living model. Most pre-clinical studies must adhere to GLPs in ICH Guidelines to be acceptable for submission to regulatory agencies such as the Food & Drug Administration in the United States.
Typically, both in vitro and in vivo tests will be performed. Studies of a drug's toxicity include which organs are targeted by that drug, as well as if there are any long-term carcinogenic effects or toxic effects on mammalian reproduction.
Animal testing
The information collected from these studies is vital so that safe human testing can begin. Typically, in drug development studies animal testing involves two species. The most commonly used models are murine and canine, although primate and porcine are also used.
Choice of species
The choice of species is based on which will give the best correlation to human trials. Differences in the gut, enzyme activity, circulatory system, or other considerations make certain models more appropriate based on the dosage form, site of activity, or noxious metabolites. For example, canines may not be good models for solid oral dosage forms because the characteristic carnivore intestine is underdeveloped compared to the omnivore's, and gastric emptying rates are increased. Also, rodents can not act as models for antibiotic drugs because the resulting alteration to their intestinal flora causes significant adverse effects. Depending on a drug's functional groups, it may be metabolized in similar or different ways between species, which will affect both efficacy and toxicology.
Medical device studies also use this basic premise. Most studies are performed in larger species such as dogs, pigs and sheep which allow for testing in a similar sized model as that of a human. In addition, some species are used for similarity in specific organs or organ system physiology (swine for dermatological and coronary stent studies; goats for mammary implant studies; dogs for gastric and cancer studies; etc.).
Ethical issues
Animal testing in the research-based pharmaceutical industry has been reduced in recent years both for ethical and cost reasons. However, most research will still involve animal based testing for the need of similarity in anatomy and physiology that is required for diverse product development.
No observable effect levels
Based on pre-clinical trials, No Observable Adverse Effect Levels (NOAEL) on drugs are established, which are used to determine initial phase 1 clinical trial dosage levels on a mass API per mass patient basis. Generally a 1/100 uncertainty factor or "safety margin" is included to account for interspecies (1/10) and inter-individual (1/10) differences.
See also
References
- ^ Ezekiel J. Emanuel. "The Solution to Drug Prices". New York Times.
On average, only one in every 5,000 compounds that drug companies discover and put through preclinical testing becomes an approved drug. Of the drugs started in clinical trials on humans, only 10 percent secure F.D.A. approval. ...