Perylenetetracarboxylic dianhydride: Difference between revisions
Appearance
Content deleted Content added
No edit summary |
use = being commercial app usually, rm specialized literature on semiconductor App Phys etc. the semiconductor stuff is academic niche |
||
| Line 29: | Line 29: | ||
}} |
}} |
||
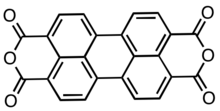
'''Perylenetetracarboxylic dianhydride''' (PTCDA) is an [[organic compound|organic]] [[dye]] molecule and an [[organic semiconductor]]. It is used as a precursor to a class of molecules known as [[Rylene dye]]s, which are useful as [[pigments]] |
'''Perylenetetracarboxylic dianhydride''' (PTCDA) is an [[organic compound|organic]] [[dye]] molecule and an [[organic semiconductor]]. It is used as a precursor to a class of molecules known as [[Rylene dye]]s, which are useful as [[pigments]] and [[dyes]]. It is a dark red solid with low solubility in aromatic solvents. The compound has attracted much interest as an [[organic semiconductor]]. |
||
== Structure == |
== Structure == |
||
| Line 43: | Line 43: | ||
|} |
|} |
||
===Self-assembly and films=== |
|||
== Uses == |
|||
[[File:PTCDA AFM.jpg|thumb|left|[[Atomic force microscopy]] image of a single PTCDA molecule on Si at room temperature.<ref name="PTCDA on Si">{{cite journal|doi=10.1038/ncomms8766|pmid=26178193|pmc=4518281|title=Chemical structure imaging of a single molecule by atomic force microscopy at room temperature|journal=Nature Communications|volume=6|pages=7766|year=2015|last1=Iwata|first1=Kota|last2=Yamazaki|first2=Shiro|last3=Mutombo|first3=Pingo|last4=Hapala|first4=Prokop|last5=Ondráček|first5=Martin|last6=Jelínek|first6=Pavel|last7=Sugimoto|first7=Yoshiaki}}</ref>]] |
[[File:PTCDA AFM.jpg|thumb|left|[[Atomic force microscopy]] image of a single PTCDA molecule on Si at room temperature.<ref name="PTCDA on Si">{{cite journal|doi=10.1038/ncomms8766|pmid=26178193|pmc=4518281|title=Chemical structure imaging of a single molecule by atomic force microscopy at room temperature|journal=Nature Communications|volume=6|pages=7766|year=2015|last1=Iwata|first1=Kota|last2=Yamazaki|first2=Shiro|last3=Mutombo|first3=Pingo|last4=Hapala|first4=Prokop|last5=Ondráček|first5=Martin|last6=Jelínek|first6=Pavel|last7=Sugimoto|first7=Yoshiaki}}</ref>]] |
||
[[File:PTCDA self-assembly STM.jpg|thumb|left|[[Self-assembly]] of PTCDA molecules on NaCl, [[scanning tunneling microscopy]] image.<ref name="PTCDA on NaCL">{{cite journal|doi=10.1038/ncomms9312|pmid=26440933|pmc=4600718|title=Pronounced polarization-induced energy level shifts at boundaries of organic semiconductor nanostructures|journal=Nature Communications|volume=6|pages=8312|year=2015|last1=Cochrane|first1=K. A.|last2=Schiffrin|first2=A.|last3=Roussy|first3=T. S.|last4=Capsoni|first4=M.|last5=Burke|first5=S. A.}}</ref>]] |
[[File:PTCDA self-assembly STM.jpg|thumb|left|[[Self-assembly]] of PTCDA molecules on NaCl, [[scanning tunneling microscopy]] image.<ref name="PTCDA on NaCL">{{cite journal|doi=10.1038/ncomms9312|pmid=26440933|pmc=4600718|title=Pronounced polarization-induced energy level shifts at boundaries of organic semiconductor nanostructures|journal=Nature Communications|volume=6|pages=8312|year=2015|last1=Cochrane|first1=K. A.|last2=Schiffrin|first2=A.|last3=Roussy|first3=T. S.|last4=Capsoni|first4=M.|last5=Burke|first5=S. A.}}</ref>]] |
||
==Use== |
|||
=== As a chemical precursor === |
|||
The main industrial use of PTCDA is as a precursor to [[Rylene dye]]s.<ref name=Ullmann1>Hunger, K. and Herbst, W. (2012) "Pigments, Organic" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. {{DOI|10.1002/14356007.a20_371}}</ref><ref>Greene, M. (2009) "Perylene Pigments", pp. 261–274 in High Performance Pigments, Wiley-VCH, Weinheim.{{DOI|10.1002/9783527626915.ch16}}</ref> |
The main industrial use of PTCDA is as a precursor to [[Rylene dye]]s.<ref name=Ullmann1>Hunger, K. and Herbst, W. (2012) "Pigments, Organic" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. {{DOI|10.1002/14356007.a20_371}}</ref><ref>Greene, M. (2009) "Perylene Pigments", pp. 261–274 in High Performance Pigments, Wiley-VCH, Weinheim.{{DOI|10.1002/9783527626915.ch16}}</ref> |
||
=== As an organic semiconductor === |
|||
PTCDA's relatively simple chemical structure makes it attractive as a model compound for the study of organic semiconductors. It has among others been used to create single-crystal [[OFET]]s<ref name="NguyenKumar Pradhan2013">{{cite journal|last1=Nguyen|first1=Linh-Nam|last2=Kumar Pradhan|first2=Sunil|last3=Yen|first3=Chia-Nan|last4=Lin|first4=Ming-Chou|last5=Chen|first5=Chien-Han|last6=Wu|first6=Cen-Shawn|last7=Chang-Liao|first7=Kuei-Shu|last8=Lin|first8=Minn-Tsong|last9=Chen|first9=Chii-Dong|title=High performance phototransistors based on single crystalline perylene-tetracarboxylic-dianhydride nanoparticle|journal=Applied Physics Letters|volume=103|issue=18|year=2013|pages=183301|issn=00036951|doi=10.1063/1.4827975}}</ref> and [[organic solar cell]]s<ref name="KumarChaudhary2014">{{cite journal|last1=Kumar|first1=Lokendra|last2=Chaudhary|first2=Dhirendra K.|title=Studies on Photovoltaic Properties of ZnPc/PTCDA Based Bilayer Organic Solar Cells|journal=Advanced Science Letters|volume=20|issue=7|year=2014|pages=1515–1518|issn=19366612|doi=10.1166/asl.2014.5728}}</ref>. It is also often used in the study of the interaction between organic semiconductors and the surfaces of [[metals]]<ref name="Tautz2007">{{cite journal|last1=Tautz|first1=F.S.|title=Structure and bonding of large aromatic molecules on noble metal surfaces: The example of PTCDA|journal=Progress in Surface Science|volume=82|issue=9-12|year=2007|pages=479–520|issn=00796816|doi=10.1016/j.progsurf.2007.09.001}}</ref>, [[insulators]]<ref name="PTCDA on NaCL"/> and [[inorganic]] [[semiconductors]]<ref name="PTCDA on Si"/>. The application of PTCDA as an organic semiconductor is limited by it's low solubility in common solvents<ref name="RussellBlunt2010">{{cite journal|last1=Russell|first1=James C.|last2=Blunt|first2=Matthew O.|last3=Goretzki|first3=Gudrun|last4=Phillips|first4=Anna G.|last5=Champness|first5=Neil R.|last6=Beton|first6=Peter H.|title=Solubilized Derivatives of Perylenetetracarboxylic Dianhydride (PTCDA) Adsorbed on Highly Oriented Pyrolytic Graphite|journal=Langmuir|volume=26|issue=6|year=2010|pages=3972–3974|issn=0743-7463|doi=10.1021/la903335v}}</ref>, which makes it unattractive for most industrial applications. |
|||
== References == |
== References == |
||
Revision as of 13:34, 8 January 2016
| |

| |
| Names | |
|---|---|
| Other names
Perylene-3,4,9,10-tetracarboxylic dianhydride, Pigment Red 224
| |
| Identifiers | |
| ECHA InfoCard | 100.004.461 |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C24H8O6 | |
| Molar mass | 392.32 |
| Density | 1.7 g/cm3 |
| Melting point | ~350 °C[1] |
| Structure | |
| Monoclinic, P21/c | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Perylenetetracarboxylic dianhydride (PTCDA) is an organic dye molecule and an organic semiconductor. It is used as a precursor to a class of molecules known as Rylene dyes, which are useful as pigments and dyes. It is a dark red solid with low solubility in aromatic solvents. The compound has attracted much interest as an organic semiconductor.
Structure
PTCDA consists of a perylene core to which two anhydride groups have been attached, one at either side. It occurs in two crystalline forms, α and β[2]. Both have the P21/c monoclinic symmetry and a density of ca. 1.7 g/cm3, which is relatively high for organic compounds. Their lattice parameters are:
| Form | a | b | c | γ |
|---|---|---|---|---|
| α | 0.374 nm | 1.196 nm | 1.734 nm | 98.8° |
| β | 0.378 nm | 1.930 nm | 1.077 nm | 83.6° |
Self-assembly and films


Use
The main industrial use of PTCDA is as a precursor to Rylene dyes.[5][6]
References
Wikimedia Commons has media related to PTCDA.
- ^ PTCDA.
- ^ Möbus, M. and Karl, N. (1992). "Structure of perylene-tetracarboxylic-dianhydride thin films on alkali halide crystal substrates". Journal of Crystal Growth. 116 (3–4): 495–504. doi:10.1016/0022-0248(92)90658-6.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Iwata, Kota; Yamazaki, Shiro; Mutombo, Pingo; Hapala, Prokop; Ondráček, Martin; Jelínek, Pavel; Sugimoto, Yoshiaki (2015). "Chemical structure imaging of a single molecule by atomic force microscopy at room temperature". Nature Communications. 6: 7766. doi:10.1038/ncomms8766. PMC 4518281. PMID 26178193.
- ^ Cochrane, K. A.; Schiffrin, A.; Roussy, T. S.; Capsoni, M.; Burke, S. A. (2015). "Pronounced polarization-induced energy level shifts at boundaries of organic semiconductor nanostructures". Nature Communications. 6: 8312. doi:10.1038/ncomms9312. PMC 4600718. PMID 26440933.
- ^ Hunger, K. and Herbst, W. (2012) "Pigments, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. doi:10.1002/14356007.a20_371
- ^ Greene, M. (2009) "Perylene Pigments", pp. 261–274 in High Performance Pigments, Wiley-VCH, Weinheim.doi:10.1002/9783527626915.ch16

