Homotaurine: Difference between revisions
Talk discussion does not go in article! And please stop forcing in content, and finish the discussion on the talk page. We talk about things here. Please talk. |
Talk about , dont erase |
||
| Line 53: | Line 53: | ||
It is a [[GABA]] antagonist, apparently by mimicking GABA, which is it resembles.<ref name=OrgChem2007/> Homotaurine has shown activity in animal models of alcoholism, and [[acamprosate]], the N-acetyl derivative of homotaurine, was approved by the FDA in 2004 to treat alcohol dependence.<ref name=OrgChem2007>Daniel Lednicer, The Organic Chemistry of Drug Synthesis. John Wiley & Sons, 2007 ISBN 9780470180662. [https://books.google.com/books?id=N6OAhuiHqiIC&pg=PA15 Page 15]</ref> |
It is a [[GABA]] antagonist, apparently by mimicking GABA, which is it resembles.<ref name=OrgChem2007/> Homotaurine has shown activity in animal models of alcoholism, and [[acamprosate]], the N-acetyl derivative of homotaurine, was approved by the FDA in 2004 to treat alcohol dependence.<ref name=OrgChem2007>Daniel Lednicer, The Organic Chemistry of Drug Synthesis. John Wiley & Sons, 2007 ISBN 9780470180662. [https://books.google.com/books?id=N6OAhuiHqiIC&pg=PA15 Page 15]</ref> |
||
Evidence says that Homotaurine is a [[GABAA receptor|GABA<sub>A</sub>]] partial agonist <ref>{{cite journal|title=Modulation of GABA-A receptors of astrocytes and STC-1 cells by taurine structural analogs.|pmid=25119985|author=Reyes-Haro D1, Cabrera-Ruíz E, Estrada-Mondragón A, Miledi R, Martínez-Torres A.}}</ref> and a [[GABAB receptor|GABA<sub>B</sub>]] receptor partial agonist with low efficacy, becoming an antagonist and displacing full agonist as [[Gamma-Aminobutyric acid|GABA]] or [[baclofen]] <ref>{{cite journal|title=Homotaurine: a GABAB antagonist in guinea-pig ileum.|pmid=6652358|author=Giotti A, Luzzi S, Spagnesi S, Zilletti L.}}</ref>. In one study, Homotaurine reversed the catatonia induced by [[baclofen]] in rats <ref>{{cite journal|title=Baclofen induces catatonia in rats.|pmid=2823166|author=Mehta AK1, Ticku MK.}}</ref> . Homotaurine produces analgesia via [[GABAB receptor|GABA<sub>B</sub>]] receptor, effect that is abolished when applied CGP 35348, a [[GABAB receptor|GABA<sub>B</sub>]] receptor antagonist <ref>{{cite journal|title=GABA(B) receptors and opioid mechanisms involved in homotaurine-induced analgesia.|pmid=9510095|author=Serrano MI1, Serrano JS, Fernández A, Asadi I, Serrano-Martino MC.}}</ref><ref>{{cite journal|title=Role of K+ -channels in homotaurine-induced analgesia.|pmid=11468027|author=Serrano MI1, Serrano JS, Asadi I, Fernández A, Serrano-Martino MC.}}</ref> . |
|||
{{multiple image |
|||
Moreover, Homotaurine suppress ethanol-stimulated dopamine release, ethanol intake and preference in rats similar to [[acamprosate]] (Acetylated homotaurine analogue) <ref>{{cite journal|title=Effects of acute acamprosate and homotaurine on ethanol intake and ethanol-stimulated mesolimbic dopamine release.|pmid=11864639|author=Olive MF, Nannini MA, Ou CJ, Koenig HN, Hodge CW.}}</ref><ref>{{cite journal|title=Acamprosate reduces ethanol drinking behaviors and alters the metabolite profile in mice lacking ENT1.|pmid=21172405|author=Berton F, et al. Alcohol Clin Exp Res. 1998. .}}</ref>. [[Acamprosate]], enhance NMDA currents and inhibited [[GABAB receptor|GABA<sub>B</sub>]] receptor too. <ref>{{cite journal|title=Acamprosate enhances N-methyl-D-apartate receptor-mediated neurotransmission but inhibits presynaptic GABA(B) receptors in nucleus accumbens neurons.|pmid=9514305|author=Olive MF, Nannini MA, Ou CJ, Koenig HN, Hodge CW.}}</ref>{{multiple image |
|||
| align = left |
| align = left |
||
| direction = vertical |
| direction = vertical |
||
Revision as of 20:01, 10 March 2016
| |
| |
| Names | |
|---|---|
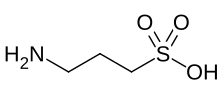
| IUPAC name
3-Aminopropane-1-sulfonic acid
| |
| Other names
Tramiprosate; Alzhemed; 3-APS
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.020.889 |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
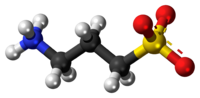
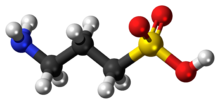
| C3H9NO3S | |
| Molar mass | 139.17 g·mol−1 |
| Melting point | 293 °C (559 °F; 566 K) (decomposition) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Homotaurine (3-amino-1-propanesulfonic acid (3-APS) or tramiprosate (INN)) is a synthetic organic compound. It is analogous to taurine, but with an extra carbon in its chain. Because of its similarity in structure to the neurotransmitter gamma-aminobutyric acid (GABA), it has GABAergic effects and may be useful as an anticonvulsant.[2]
Homotaurine was investigated in a Phase III clinical trial as a potential treatment for Alzheimer's disease that did not show efficacy in its primary endpoints.[3] In preclinical studies it had been found to bind to soluble amyloid beta and inhibit the formation of neurotoxic aggregates.[3][4][5]
It is a GABA antagonist, apparently by mimicking GABA, which is it resembles.[6] Homotaurine has shown activity in animal models of alcoholism, and acamprosate, the N-acetyl derivative of homotaurine, was approved by the FDA in 2004 to treat alcohol dependence.[6]
Evidence says that Homotaurine is a GABAA partial agonist [7] and a GABAB receptor partial agonist with low efficacy, becoming an antagonist and displacing full agonist as GABA or baclofen [8]. In one study, Homotaurine reversed the catatonia induced by baclofen in rats [9] . Homotaurine produces analgesia via GABAB receptor, effect that is abolished when applied CGP 35348, a GABAB receptor antagonist [10][11] .
Moreover, Homotaurine suppress ethanol-stimulated dopamine release, ethanol intake and preference in rats similar to acamprosate (Acetylated homotaurine analogue) [12][13]. Acamprosate, enhance NMDA currents and inhibited GABAB receptor too. [14]
References
- ^ Homotaurine at Sigma-Aldrich
- ^ Fariello RG, Golden GT, Pisa M; Golden; Pisa (1982). "Homotaurine (3 aminopropanesulfonic acid; 3APS) protects from the convulsant and cytotoxic effect of systemically administered kainic acid". Neurology. 32 (3): 241–5. doi:10.1212/wnl.32.3.241. PMID 7199633.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Caltagirone C et al. The potential protective effect of tramiprosate (homotaurine) against Alzheimer's disease: a review. Aging Clin Exp Res. 2012 Dec;24(6):580-7. PMID 22961121
- ^ Herrmann N et al. Current and emerging drug treatment options for Alzheimer's disease: a systematic review. Drugs. 2011 Oct 22;71(15):2031-65. Review. PMID 21985169
- ^ Aisen PS Alzhemed: a potential treatment for Alzheimer's disease. Curr Alzheimer Res. 2007 Sep;4(4):473-8. PMID 17908052
- ^ a b Daniel Lednicer, The Organic Chemistry of Drug Synthesis. John Wiley & Sons, 2007 ISBN 9780470180662. Page 15
- ^ Reyes-Haro D1, Cabrera-Ruíz E, Estrada-Mondragón A, Miledi R, Martínez-Torres A. "Modulation of GABA-A receptors of astrocytes and STC-1 cells by taurine structural analogs". PMID 25119985.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Giotti A, Luzzi S, Spagnesi S, Zilletti L. "Homotaurine: a GABAB antagonist in guinea-pig ileum". PMID 6652358.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ^ Mehta AK1, Ticku MK. "Baclofen induces catatonia in rats". PMID 2823166.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: numeric names: authors list (link) - ^ Serrano MI1, Serrano JS, Fernández A, Asadi I, Serrano-Martino MC. "GABA(B) receptors and opioid mechanisms involved in homotaurine-induced analgesia". PMID 9510095.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Serrano MI1, Serrano JS, Asadi I, Fernández A, Serrano-Martino MC. "Role of K+ -channels in homotaurine-induced analgesia". PMID 11468027.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Olive MF, Nannini MA, Ou CJ, Koenig HN, Hodge CW. "Effects of acute acamprosate and homotaurine on ethanol intake and ethanol-stimulated mesolimbic dopamine release". PMID 11864639.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ^ Berton F, et al. Alcohol Clin Exp Res. 1998. . "Acamprosate reduces ethanol drinking behaviors and alters the metabolite profile in mice lacking ENT1". PMID 21172405.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: numeric names: authors list (link) - ^ Olive MF, Nannini MA, Ou CJ, Koenig HN, Hodge CW. "Acamprosate enhances N-methyl-D-apartate receptor-mediated neurotransmission but inhibits presynaptic GABA(B) receptors in nucleus accumbens neurons". PMID 9514305.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link)