Damascenone: Difference between revisions
Appearance
Content deleted Content added
Tom.Reding (talk | contribs) m CS1 maintenance: vauthors/veditors or enumerate multiple authors/editors; WP:GenFixes on using AWB |
No edit summary |
||
| Line 67: | Line 67: | ||
| url = http://pubs.acs.org/doi/full/10.1021/jf800382m |
| url = http://pubs.acs.org/doi/full/10.1021/jf800382m |
||
| doi = 10.1021/jf800382m }}</ref> |
| doi = 10.1021/jf800382m }}</ref> |
||
==Biosynthesis== |
|||
==See also== |
==See also== |
||
Revision as of 00:31, 24 May 2016
| |

| |
| Names | |
|---|---|
| IUPAC name
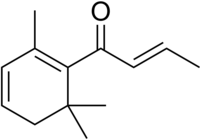
(E)-1-(2,6,6-Trimethyl-1-cyclohexa-1,3-dienyl)but-2-en-1-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.041.662 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H18O | |
| Molar mass | 190.28 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Damascenones are a series of closely related chemical compounds that are components of a variety of essential oils. The damascenones belong to a family of chemicals known as rose ketones, which also includes damascones and ionones. beta-Damascenone is a major contributor to the aroma of roses, despite its very low concentration, and is an important fragrance chemical used in perfumery.[1]
The damascenones are derived from the degradation of carotenoids.[2]
In 2008, (E)-β-damascenone was identified as a primary odorant in Kentucky Bourbon.[3]
Biosynthesis
See also
References
- ^ Rose (Rosa damascena), John C. Leffingwell
- ^ Sachihiko Isoe; Shigeo Katsumura; Takeo Sakan (1973). "The Synthesis of Damascenone and beta-Damascone and the possible mechanism of their formation from carotenoids". Helvetica Chimica Acta. 56 (5): 1514–1516. doi:10.1002/hlca.19730560508.
- ^ LUIGI POISSON; PETER SCHIEBERLE (2008). "Characterization of the Most Odor-Active Compounds in an American Bourbon Whisky by Application of the Aroma Extract Dilution Analysis". J. Agric. Food Chem. 56 (14): 5813–5819. doi:10.1021/jf800382m.
