STK11: Difference between revisions
Updating to new gene infobox populated via wikidata |
m clean up using AWB |
||
| Line 1: | Line 1: | ||
{{Infobox_gene}} |
{{Infobox_gene}} |
||
'''Serine/threonine kinase 11''' (STK11) also known as '''liver kinase B1''' (LKB1) or '''renal carcinoma antigen NY-REN-19''' is a [[protein]] [[kinase]] that in humans is encoded by the '''''STK11''''' [[gene]].<ref name="pmid9425897">{{cite journal | |
'''Serine/threonine kinase 11''' (STK11) also known as '''liver kinase B1''' (LKB1) or '''renal carcinoma antigen NY-REN-19''' is a [[protein]] [[kinase]] that in humans is encoded by the '''''STK11''''' [[gene]].<ref name="pmid9425897">{{cite journal |vauthors=Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, Müller O, Back W, Zimmer M | title = Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase | journal = Nature Genetics | volume = 18 | issue = 1 | pages = 38–43 |date=January 1998 | pmid = 9425897 | doi = 10.1038/ng0198-38 | url = }}</ref> |
||
==Expression== |
==Expression== |
||
[[Testosterone]] and [[Dihydrotestosterone|DHT]] treatment of murine 3T3-L1 or human SGBS adipocytes for 24 h significantly decreased the mRNA expression of LKB1 via the androgen receptor and consequently reduced the activation of [[AMP-activated protein kinase|AMPK]] by phosphorylation. In contrast, [[17β-estradiol]] treatment increased LKB1 mRNA, an effect mediated by oestrogen receptor alpha.<ref>{{cite journal | doi = 10.1038/ijo.2011.172 | pmid = 21876548 | title=Regulation of LKB1 expression by sex hormones in adipocytes |date=August 2011 | journal=Int J Obes (Lond) | |
[[Testosterone]] and [[Dihydrotestosterone|DHT]] treatment of murine 3T3-L1 or human SGBS adipocytes for 24 h significantly decreased the mRNA expression of LKB1 via the androgen receptor and consequently reduced the activation of [[AMP-activated protein kinase|AMPK]] by phosphorylation. In contrast, [[17β-estradiol]] treatment increased LKB1 mRNA, an effect mediated by oestrogen receptor alpha.<ref>{{cite journal | doi = 10.1038/ijo.2011.172 | pmid = 21876548 | title=Regulation of LKB1 expression by sex hormones in adipocytes |date=August 2011 | journal=Int J Obes (Lond) |vauthors=McInnes KJ, Brown KA, Hunger NI, Simpson ER | volume=36 | pages=982–985}}</ref> |
||
However, in ER-positive breast cancer cell line MCF-7, estradiol caused a dose-dependent decrease in LKB1 transcript and protein expression leading to a significant decrease in the phosphorylation of the LKB1 target AMPK. [[ESR1|ERα]] binds to the STK11 promoter in a ligand-independent manner and this interaction is decreased in the presence of estradiol. Moreover, STK11 promoter activity is significantly decreased in the presence of estradiol.<ref name="pmid21689749">{{cite journal | |
However, in ER-positive breast cancer cell line MCF-7, estradiol caused a dose-dependent decrease in LKB1 transcript and protein expression leading to a significant decrease in the phosphorylation of the LKB1 target AMPK. [[ESR1|ERα]] binds to the STK11 promoter in a ligand-independent manner and this interaction is decreased in the presence of estradiol. Moreover, STK11 promoter activity is significantly decreased in the presence of estradiol.<ref name="pmid21689749">{{cite journal |vauthors=Brown KA, McInnes KJ, Takagi K, Ono K, Hunger NI, Wang L, Sasano H, Simpson ER | title = LKB1 expression is inhibited by estradiol-17β in MCF-7 cells | journal = J. Steroid Biochem. Mol. Biol. | volume = 127 | issue = 3-5 | pages = 439–43 |date=November 2011 | pmid = 21689749 | doi = 10.1016/j.jsbmb.2011.06.005 }}</ref> |
||
== Function == |
== Function == |
||
| Line 13: | Line 13: | ||
== Clinical significance == |
== Clinical significance == |
||
[[Germline]] [[mutations]] in this gene have been associated with [[Peutz-Jeghers syndrome]], an [[autosomal dominant]] disorder characterized by the growth of [[polyps]] in the gastrointestinal tract, pigmented [[macules]] on the skin and mouth, and other [[neoplasms]].<ref name="pmid8988175">{{cite journal | |
[[Germline]] [[mutations]] in this gene have been associated with [[Peutz-Jeghers syndrome]], an [[autosomal dominant]] disorder characterized by the growth of [[polyps]] in the gastrointestinal tract, pigmented [[macules]] on the skin and mouth, and other [[neoplasms]].<ref name="pmid8988175">{{cite journal |vauthors=Hemminki A, Tomlinson I, Markie D, Järvinen H, Sistonen P, Björkqvist AM, Knuutila S, Salovaara R, Bodmer W, Shibata D, de la Chapelle A, Aaltonen LA | title = Localization of a susceptibility locus for Peutz-Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis | journal = Nat. Genet. | volume = 15 | issue = 1 | pages = 87–90 |date=January 1997 | pmid = 8988175 | doi = 10.1038/ng0197-87 }}</ref><ref name="pmid9428765">{{cite journal |vauthors=Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Höglund P, Järvinen H, Kristo P, Pelin K, Ridanpää M, Salovaara R, Toro T, Bodmer W, Olschwang S, Olsen AS, Stratton MR, de la Chapelle A, Aaltonen LA | title = A serine/threonine kinase gene defective in Peutz-Jeghers syndrome | journal = Nature | volume = 391 | issue = 6663 | pages = 184–7 |date=January 1998 | pmid = 9428765 | doi = 10.1038/34432 }}</ref><ref name="pmid18846624">{{cite journal |vauthors=Scott R, Crooks R, Meldrum C | title = Gene symbol: STK11. Disease: Peutz-Jeghers Syndrome | journal = Human Genetics | volume = 124 | issue = 3 | pages = 300 |date=October 2008 | pmid = 18846624 | doi = 10.1007/s00439-008-0551-3 }}</ref> However, the LKB1 gene was also found to be mutated in lung cancer of sporadic origin, predominantly adenocarcinomas.<ref name="pmid= 12097271">{{cite journal |vauthors=Sanchez-Cespedes M, Parrella P, Esteller M, Nomoto S, Trink B, Engles JM, Westra WH, Herman JG, Sidransky D |title= Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. |journal= Cancer Res. |volume=62 |issue= 13 |pages= 3659–62 |year= 2002 |pmid= 12097271 }}</ref> Further, more recent studies have uncovered a large number of somatic mutations of the LKB1 gene that are present in cervical, breast, intestinal, testicular, pancreatic and skin cancer.<ref name="pmid= 17599048">{{cite journal | author=Sanchez-Cespedes M' |title= A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome. |journal=Oncogene |volume=26 |pages= 7825–32 |year= 2007 |pmid= 17599048 | doi=10.1038/sj.onc.1210594 | issue=57 }}</ref><ref name="urlCatalogue of Somatic Mutations in Cancer">{{cite web | url = http://www.sanger.ac.uk/perl/genetics/CGP/cosmic?action=bygene&ln=STK11&start=1&end=434&coords=AA:AA | title = Distribution of somatic mutations in STK11 | work = Catalogue of Somatic Mutations in Cancer | publisher = Wellcome Trust Genome Campus, Hinxton, Cambridge | accessdate = 2009-11-11 }}</ref> |
||
==Activation== |
==Activation== |
||
| Line 19: | Line 19: | ||
==Structure== |
==Structure== |
||
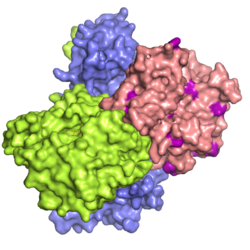
The crystal structure of the LKB1-STRAD-MO25 complex was elucidated using [[X-ray crystallography]],<ref name="pmid19892943">{{PDB|2WTK}}; {{cite journal | |
The crystal structure of the LKB1-STRAD-MO25 complex was elucidated using [[X-ray crystallography]],<ref name="pmid19892943">{{PDB|2WTK}}; {{cite journal |vauthors=Zeqiraj E, Filippi BM, Deak M, Alessi DR, van Aalten DM | title = Structure of the LKB1-STRAD-MO25 Complex Reveals an Allosteric Mechanism of Kinase Activation | journal = Science | volume = 326| issue = 5960| pages = 1707–11|date=November 2009 | pmid = 19892943 | doi = 10.1126/science.1178377 | url = | issn = | pmc=3518268}}</ref> and revealed the mechanism by which LKB1 is [[allosterically]] activated. LKB1 has a structure typical of other protein [[kinases]], with two (small and large) lobes on either side of the ligand [[Adenosine triphosphate|ATP]]-binding pocket. [[LYK5|STRAD]] and [[MO25]] together cooperate to promote LKB1 active conformation. The LKB1 activation loop, a critical element in the process of [[kinase]] activation, is held in place by [[MO25]], thus explaining the huge increase in LKB1 activity in the presence of [[LYK5|STRAD]] and [[MO25]] . |
||
== Splice variants == |
== Splice variants == |
||
| Line 27: | Line 27: | ||
STK11 has been shown to [[Protein-protein interaction|interact]] with: |
STK11 has been shown to [[Protein-protein interaction|interact]] with: |
||
{{div col|colwidth=20em}} |
{{div col|colwidth=20em}} |
||
* [[CDC37]],<ref name = pmid12489981>{{cite journal | |
* [[CDC37]],<ref name = pmid12489981>{{cite journal |vauthors=Boudeau J, Deak M, Lawlor MA, Morrice NA, Alessi DR | title = Heat-shock protein 90 and Cdc37 interact with LKB1 and regulate its stability | journal = Biochem. J. | volume = 370 | issue = Pt 3 | pages = 849–57 | year = 2003 | pmid = 12489981 | pmc = 1223241 | doi = 10.1042/BJ20021813 }}</ref> |
||
* * [[Fyn]]<ref name=pmid24586906>{{cite journal |last=Yamada |first=Eijiro |authorlink= |author2=Claire C. Bastie |date=February 2014 |title=Disruption of Fyn SH3 Domain Interaction with a Proline-Rich Motif in Liver Kinase B1 Results in Activation of AMP-Activated Protein Kinase. |journal=PLOS ONE |volume=9 |issue=2 |pages=e89604 |publisher= |location= United States| doi = 10.1371/journal.pone.0089604 | bibcode = | oclc =| id = | url = | language = | format = | accessdate = | laysummary = | laysource = | laydate = | quote = | pmid=24586906 | pmc=3934923}}</ref> |
* * [[Fyn]]<ref name=pmid24586906>{{cite journal |last=Yamada |first=Eijiro |authorlink= |author2=Claire C. Bastie |date=February 2014 |title=Disruption of Fyn SH3 Domain Interaction with a Proline-Rich Motif in Liver Kinase B1 Results in Activation of AMP-Activated Protein Kinase. |journal=PLOS ONE |volume=9 |issue=2 |pages=e89604 |publisher= |location= United States| doi = 10.1371/journal.pone.0089604 | bibcode = | oclc =| id = | url = | language = | format = | accessdate = | laysummary = | laysource = | laydate = | quote = | pmid=24586906 | pmc=3934923}}</ref> |
||
* [[Heat shock protein 90kDa alpha (cytosolic), member A1|HSP90AA1]]<ref name = pmid12489981/> |
* [[Heat shock protein 90kDa alpha (cytosolic), member A1|HSP90AA1]]<ref name = pmid12489981/> |
||
* [[LYK5]],<ref name = pmid15561763>{{cite journal | |
* [[LYK5]],<ref name = pmid15561763>{{cite journal |vauthors=Boudeau J, Scott JW, Resta N, Deak M, Kieloch A, Komander D, Hardie DG, Prescott AR, van Aalten DM, Alessi DR | title = Analysis of the LKB1-STRAD-MO25 complex | journal = J. Cell. Sci. | volume = 117 | issue = Pt 26 | pages = 6365–75 | year = 2004 | pmid = 15561763 | doi = 10.1242/jcs.01571 }}</ref><ref name = pmid12805220>{{cite journal |vauthors=Baas AF, Boudeau J, Sapkota GP, Smit L, Medema R, Morrice NA, Alessi DR, Clevers HC | title = Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD | journal = EMBO J. | volume = 22 | issue = 12 | pages = 3062–72 | year = 2003 | pmid = 12805220 | pmc = 162144 | doi = 10.1093/emboj/cdg292 }}</ref> and |
||
* [[SMARCA4]].<ref name = pmid11445556>{{cite journal | |
* [[SMARCA4]].<ref name = pmid11445556>{{cite journal |vauthors=Marignani PA, Kanai F, Carpenter CL | title = LKB1 associates with Brg1 and is necessary for Brg1-induced growth arrest | journal = J. Biol. Chem. | volume = 276 | issue = 35 | pages = 32415–8 | year = 2001 | pmid = 11445556 | doi = 10.1074/jbc.C100207200 }}</ref> |
||
* [[ERalpha]] |
* [[ERalpha]] |
||
{{Div col end}} |
{{Div col end}} |
||
| Line 41: | Line 41: | ||
==Further reading== |
==Further reading== |
||
{{Refbegin|35em}} |
{{Refbegin|35em}} |
||
*{{cite journal | |
*{{cite journal |vauthors=Yoo LI, Chung DC, Yuan J |title=LKB1--a master tumour suppressor of the small intestine and beyond |journal=Nat. Rev. Cancer |volume=2 |issue= 7 |pages= 529–35 |year= 2002 |pmid= 12094239 |doi= 10.1038/nrc843 }} |
||
*{{cite journal | |
*{{cite journal |vauthors=Baas AF, Smit L, Clevers H |title=LKB1 tumor suppressor protein: PARtaker in cell polarity |journal=Trends Cell Biol. |volume=14 |issue= 6 |pages= 312–9 |year= 2004 |pmid= 15183188 |doi= 10.1016/j.tcb.2004.04.001 }} |
||
*{{cite journal |vauthors=Katajisto P, Vallenius T, Vaahtomeri K, etal |title=The LKB1 tumor suppressor kinase in human disease |journal=Biochim. Biophys. Acta |volume=1775 |issue= 1 |pages= 63–75 |year= 2007 |pmid= 17010524 |doi= 10.1016/j.bbcan.2006.08.003 }} |
*{{cite journal |vauthors=Katajisto P, Vallenius T, Vaahtomeri K, etal |title=The LKB1 tumor suppressor kinase in human disease |journal=Biochim. Biophys. Acta |volume=1775 |issue= 1 |pages= 63–75 |year= 2007 |pmid= 17010524 |doi= 10.1016/j.bbcan.2006.08.003 }} |
||
*{{cite journal | |
*{{cite journal |vauthors=Bonaldo MF, Lennon G, Soares MB |title=Normalization and subtraction: two approaches to facilitate gene discovery |journal=Genome Res. |volume=6 |issue= 9 |pages= 791–806 |year= 1997 |pmid= 8889548 |doi=10.1101/gr.6.9.791 }} |
||
*{{cite journal |vauthors=Hemminki A, Tomlinson I, Markie D, etal |title=Localization of a susceptibility locus for Peutz-Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis |journal=Nat. Genet. |volume=15 |issue= 1 |pages= 87–90 |year= 1997 |pmid= 8988175 |doi= 10.1038/ng0197-87 }} |
*{{cite journal |vauthors=Hemminki A, Tomlinson I, Markie D, etal |title=Localization of a susceptibility locus for Peutz-Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis |journal=Nat. Genet. |volume=15 |issue= 1 |pages= 87–90 |year= 1997 |pmid= 8988175 |doi= 10.1038/ng0197-87 }} |
||
*{{cite journal |vauthors=Jenne DE, Reimann H, Nezu J, etal |title=Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase |journal=Nat. Genet. |volume=18 |issue= 1 |pages= 38–43 |year= 1998 |pmid= 9425897 |doi= 10.1038/ng0198-38 }} |
*{{cite journal |vauthors=Jenne DE, Reimann H, Nezu J, etal |title=Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase |journal=Nat. Genet. |volume=18 |issue= 1 |pages= 38–43 |year= 1998 |pmid= 9425897 |doi= 10.1038/ng0198-38 }} |
||
| Line 55: | Line 55: | ||
*{{cite journal |vauthors=Westerman AM, Entius MM, Boor PP, etal |title=Novel mutations in the LKB1/STK11 gene in Dutch Peutz-Jeghers families |journal=Hum. Mutat. |volume=13 |issue= 6 |pages= 476–81 |year= 1999 |pmid= 10408777 |doi= 10.1002/(SICI)1098-1004(1999)13:6<476::AID-HUMU7>3.0.CO;2-2 }} |
*{{cite journal |vauthors=Westerman AM, Entius MM, Boor PP, etal |title=Novel mutations in the LKB1/STK11 gene in Dutch Peutz-Jeghers families |journal=Hum. Mutat. |volume=13 |issue= 6 |pages= 476–81 |year= 1999 |pmid= 10408777 |doi= 10.1002/(SICI)1098-1004(1999)13:6<476::AID-HUMU7>3.0.CO;2-2 }} |
||
*{{cite journal |vauthors=Scanlan MJ, Gordan JD, Williamson B, etal |title=Antigens recognized by autologous antibody in patients with renal-cell carcinoma |journal=Int. J. Cancer |volume=83 |issue= 4 |pages= 456–64 |year= 1999 |pmid= 10508479 |doi=10.1002/(SICI)1097-0215(19991112)83:4<456::AID-IJC4>3.0.CO;2-5 }} |
*{{cite journal |vauthors=Scanlan MJ, Gordan JD, Williamson B, etal |title=Antigens recognized by autologous antibody in patients with renal-cell carcinoma |journal=Int. J. Cancer |volume=83 |issue= 4 |pages= 456–64 |year= 1999 |pmid= 10508479 |doi=10.1002/(SICI)1097-0215(19991112)83:4<456::AID-IJC4>3.0.CO;2-5 }} |
||
*{{cite journal | |
*{{cite journal |vauthors=Collins SP, Reoma JL, Gamm DM, Uhler MD |title=LKB1, a novel serine/threonine protein kinase and potential tumour suppressor, is phosphorylated by cAMP-dependent protein kinase (PKA) and prenylated in vivo |journal=Biochem. J. |volume=345 |issue= 3|pages= 673–80 |year= 2000 |pmid= 10642527 |doi=10.1042/0264-6021:3450673 | pmc=1220803 }} |
||
*{{cite journal |vauthors=Sapkota GP, Kieloch A, Lizcano JM, etal |title=Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell vrowth |journal=J. Biol. Chem. |volume=276 |issue= 22 |pages= 19469–82 |year= 2001 |pmid= 11297520 |doi= 10.1074/jbc.M009953200 }} |
*{{cite journal |vauthors=Sapkota GP, Kieloch A, Lizcano JM, etal |title=Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell vrowth |journal=J. Biol. Chem. |volume=276 |issue= 22 |pages= 19469–82 |year= 2001 |pmid= 11297520 |doi= 10.1074/jbc.M009953200 }} |
||
*{{cite journal |vauthors=Karuman P, Gozani O, Odze RD, etal |title=The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death |journal=Mol. Cell |volume=7 |issue= 6 |pages= 1307–19 |year= 2001 |pmid= 11430832 |doi=10.1016/S1097-2765(01)00258-1 }} |
*{{cite journal |vauthors=Karuman P, Gozani O, Odze RD, etal |title=The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death |journal=Mol. Cell |volume=7 |issue= 6 |pages= 1307–19 |year= 2001 |pmid= 11430832 |doi=10.1016/S1097-2765(01)00258-1 }} |
||
*{{cite journal | |
*{{cite journal |vauthors=Marignani PA, Kanai F, Carpenter CL |title=LKB1 associates with Brg1 and is necessary for Brg1-induced growth arrest |journal=J. Biol. Chem. |volume=276 |issue= 35 |pages= 32415–8 |year= 2001 |pmid= 11445556 |doi= 10.1074/jbc.C100207200 }} |
||
*{{cite journal | |
*{{cite journal |vauthors=Carretero J, Medina PP, Pio R, Montuenga LM, Sanchez-Cespedes M |title= Novel and natural knockout lung cancer cell lines for the LKB1/STK11 tumor suppressor gene.|journal=Cancer Res.|volume=23|issue=22|pages= 4037–40.|year= 2004 |
||
|pmid=15021901|doi= 10.1038/sj.onc.1207502}} |
|pmid=15021901|doi= 10.1038/sj.onc.1207502}} |
||
*{{cite journal |vauthors=Abed AA, Günther K, Kraus C, etal |title=Mutation screening at the RNA level of the STK11/LKB1 gene in Peutz-Jeghers syndrome reveals complex splicing abnormalities and a novel mRNA isoform (STK11 c.597(insertion mark)598insIVS4) |journal=Hum. Mutat. |volume=18 |issue= 5 |pages= 397–410 |year= 2002 |pmid= 11668633 |doi= 10.1002/humu.1211 }} |
*{{cite journal |vauthors=Abed AA, Günther K, Kraus C, etal |title=Mutation screening at the RNA level of the STK11/LKB1 gene in Peutz-Jeghers syndrome reveals complex splicing abnormalities and a novel mRNA isoform (STK11 c.597(insertion mark)598insIVS4) |journal=Hum. Mutat. |volume=18 |issue= 5 |pages= 397–410 |year= 2002 |pmid= 11668633 |doi= 10.1002/humu.1211 }} |
||
Revision as of 17:25, 25 May 2016
Serine/threonine kinase 11 (STK11) also known as liver kinase B1 (LKB1) or renal carcinoma antigen NY-REN-19 is a protein kinase that in humans is encoded by the STK11 gene.[5]
Expression
Testosterone and DHT treatment of murine 3T3-L1 or human SGBS adipocytes for 24 h significantly decreased the mRNA expression of LKB1 via the androgen receptor and consequently reduced the activation of AMPK by phosphorylation. In contrast, 17β-estradiol treatment increased LKB1 mRNA, an effect mediated by oestrogen receptor alpha.[6]
However, in ER-positive breast cancer cell line MCF-7, estradiol caused a dose-dependent decrease in LKB1 transcript and protein expression leading to a significant decrease in the phosphorylation of the LKB1 target AMPK. ERα binds to the STK11 promoter in a ligand-independent manner and this interaction is decreased in the presence of estradiol. Moreover, STK11 promoter activity is significantly decreased in the presence of estradiol.[7]
Function
The STK11/LKB1 gene, which encodes a member of the serine/threonine kinase family, regulates cell polarity and functions as a tumour suppressor.
LKB1 is a primary upstream kinase of adenosine monophosphate-activated protein kinase (AMPK), a necessary element in cell metabolism that is required for maintaining energy homeostasis. It is now clear that LKB1 exerts its growth suppressing effects by activating a group of ~14 other kinases, comprising AMPK and AMPK-related kinases. Activation of AMPK by LKB1 suppresses growth and proliferation when energy and nutrient levels are scarce. Activation of AMPK-related kinases by LKB1 plays vital roles maintaining cell polarity thereby inhibiting inappropriate expansion of tumour cells. A picture from current research is emerging that loss of LKB1 leads to disorganization of cell polarity and facilitates tumour growth under energetically unfavorable conditions.
Clinical significance
Germline mutations in this gene have been associated with Peutz-Jeghers syndrome, an autosomal dominant disorder characterized by the growth of polyps in the gastrointestinal tract, pigmented macules on the skin and mouth, and other neoplasms.[8][9][10] However, the LKB1 gene was also found to be mutated in lung cancer of sporadic origin, predominantly adenocarcinomas.[11] Further, more recent studies have uncovered a large number of somatic mutations of the LKB1 gene that are present in cervical, breast, intestinal, testicular, pancreatic and skin cancer.[12][13]
Activation
LKB1 is activated allosterically by binding to the pseudokinase STRAD and the adaptor protein MO25. The LKB1-STRAD-MO25 heterotrimeric complex represents the biologically active unit, that is capable of phosphorylating and activating AMPK and at least 12 other kinases that belong to the AMPK-related kinase family.
Structure
The crystal structure of the LKB1-STRAD-MO25 complex was elucidated using X-ray crystallography,[14] and revealed the mechanism by which LKB1 is allosterically activated. LKB1 has a structure typical of other protein kinases, with two (small and large) lobes on either side of the ligand ATP-binding pocket. STRAD and MO25 together cooperate to promote LKB1 active conformation. The LKB1 activation loop, a critical element in the process of kinase activation, is held in place by MO25, thus explaining the huge increase in LKB1 activity in the presence of STRAD and MO25 .
Splice variants
Alternate transcriptional splice variants of this gene have been observed and characterized. There are two main splice isoforms denoted LKB1 long (LKB1L) and LKB1 short (LKB1S). The short LKB1 variant is predominantly found in testes.
Interactions
STK11 has been shown to interact with:
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000118046 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000003068 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, Müller O, Back W, Zimmer M (January 1998). "Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase". Nature Genetics. 18 (1): 38–43. doi:10.1038/ng0198-38. PMID 9425897.
- ^ McInnes KJ, Brown KA, Hunger NI, Simpson ER (August 2011). "Regulation of LKB1 expression by sex hormones in adipocytes". Int J Obes (Lond). 36: 982–985. doi:10.1038/ijo.2011.172. PMID 21876548.
- ^ Brown KA, McInnes KJ, Takagi K, Ono K, Hunger NI, Wang L, Sasano H, Simpson ER (November 2011). "LKB1 expression is inhibited by estradiol-17β in MCF-7 cells". J. Steroid Biochem. Mol. Biol. 127 (3–5): 439–43. doi:10.1016/j.jsbmb.2011.06.005. PMID 21689749.
- ^ Hemminki A, Tomlinson I, Markie D, Järvinen H, Sistonen P, Björkqvist AM, Knuutila S, Salovaara R, Bodmer W, Shibata D, de la Chapelle A, Aaltonen LA (January 1997). "Localization of a susceptibility locus for Peutz-Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis". Nat. Genet. 15 (1): 87–90. doi:10.1038/ng0197-87. PMID 8988175.
- ^ Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Höglund P, Järvinen H, Kristo P, Pelin K, Ridanpää M, Salovaara R, Toro T, Bodmer W, Olschwang S, Olsen AS, Stratton MR, de la Chapelle A, Aaltonen LA (January 1998). "A serine/threonine kinase gene defective in Peutz-Jeghers syndrome". Nature. 391 (6663): 184–7. doi:10.1038/34432. PMID 9428765.
- ^ Scott R, Crooks R, Meldrum C (October 2008). "Gene symbol: STK11. Disease: Peutz-Jeghers Syndrome". Human Genetics. 124 (3): 300. doi:10.1007/s00439-008-0551-3. PMID 18846624.
- ^ Sanchez-Cespedes M, Parrella P, Esteller M, Nomoto S, Trink B, Engles JM, Westra WH, Herman JG, Sidransky D (2002). "Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung". Cancer Res. 62 (13): 3659–62. PMID 12097271.
- ^ Sanchez-Cespedes M' (2007). "A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome". Oncogene. 26 (57): 7825–32. doi:10.1038/sj.onc.1210594. PMID 17599048.
- ^ "Distribution of somatic mutations in STK11". Catalogue of Somatic Mutations in Cancer. Wellcome Trust Genome Campus, Hinxton, Cambridge. Retrieved 2009-11-11.
- ^ PDB: 2WTK; Zeqiraj E, Filippi BM, Deak M, Alessi DR, van Aalten DM (November 2009). "Structure of the LKB1-STRAD-MO25 Complex Reveals an Allosteric Mechanism of Kinase Activation". Science. 326 (5960): 1707–11. doi:10.1126/science.1178377. PMC 3518268. PMID 19892943.
- ^ a b Boudeau J, Deak M, Lawlor MA, Morrice NA, Alessi DR (2003). "Heat-shock protein 90 and Cdc37 interact with LKB1 and regulate its stability". Biochem. J. 370 (Pt 3): 849–57. doi:10.1042/BJ20021813. PMC 1223241. PMID 12489981.
- ^ Yamada, Eijiro; Claire C. Bastie (February 2014). "Disruption of Fyn SH3 Domain Interaction with a Proline-Rich Motif in Liver Kinase B1 Results in Activation of AMP-Activated Protein Kinase". PLOS ONE. 9 (2). United States: e89604. doi:10.1371/journal.pone.0089604. PMC 3934923. PMID 24586906.
{{cite journal}}: Cite has empty unknown parameters:|laysummary=,|laydate=, and|laysource=(help)CS1 maint: unflagged free DOI (link) - ^ Boudeau J, Scott JW, Resta N, Deak M, Kieloch A, Komander D, Hardie DG, Prescott AR, van Aalten DM, Alessi DR (2004). "Analysis of the LKB1-STRAD-MO25 complex". J. Cell. Sci. 117 (Pt 26): 6365–75. doi:10.1242/jcs.01571. PMID 15561763.
- ^ Baas AF, Boudeau J, Sapkota GP, Smit L, Medema R, Morrice NA, Alessi DR, Clevers HC (2003). "Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD". EMBO J. 22 (12): 3062–72. doi:10.1093/emboj/cdg292. PMC 162144. PMID 12805220.
- ^ Marignani PA, Kanai F, Carpenter CL (2001). "LKB1 associates with Brg1 and is necessary for Brg1-induced growth arrest". J. Biol. Chem. 276 (35): 32415–8. doi:10.1074/jbc.C100207200. PMID 11445556.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
Further reading
- Yoo LI, Chung DC, Yuan J (2002). "LKB1--a master tumour suppressor of the small intestine and beyond". Nat. Rev. Cancer. 2 (7): 529–35. doi:10.1038/nrc843. PMID 12094239.
- Baas AF, Smit L, Clevers H (2004). "LKB1 tumor suppressor protein: PARtaker in cell polarity". Trends Cell Biol. 14 (6): 312–9. doi:10.1016/j.tcb.2004.04.001. PMID 15183188.
- Katajisto P, Vallenius T, Vaahtomeri K, et al. (2007). "The LKB1 tumor suppressor kinase in human disease". Biochim. Biophys. Acta. 1775 (1): 63–75. doi:10.1016/j.bbcan.2006.08.003. PMID 17010524.
- Bonaldo MF, Lennon G, Soares MB (1997). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Hemminki A, Tomlinson I, Markie D, et al. (1997). "Localization of a susceptibility locus for Peutz-Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis". Nat. Genet. 15 (1): 87–90. doi:10.1038/ng0197-87. PMID 8988175.
- Jenne DE, Reimann H, Nezu J, et al. (1998). "Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase". Nat. Genet. 18 (1): 38–43. doi:10.1038/ng0198-38. PMID 9425897.
- Hemminki A, Markie D, Tomlinson I, et al. (1998). "A serine/threonine kinase gene defective in Peutz-Jeghers syndrome". Nature. 391 (6663): 184–7. doi:10.1038/34432. PMID 9428765.
- Bignell GR, Barfoot R, Seal S, et al. (1998). "Low frequency of somatic mutations in the LKB1/Peutz-Jeghers syndrome gene in sporadic breast cancer". Cancer Res. 58 (7): 1384–6. PMID 9537235.
- Nakagawa H, Koyama K, Miyoshi Y, et al. (1998). "Nine novel germline mutations of STK11 in ten families with Peutz-Jeghers syndrome". Hum. Genet. 103 (2): 168–72. doi:10.1007/s004390050801. PMID 9760200.
- Mehenni H, Gehrig C, Nezu J, et al. (1999). "Loss of LKB1 kinase activity in Peutz-Jeghers syndrome, and evidence for allelic and locus heterogeneity". Am. J. Hum. Genet. 63 (6): 1641–50. doi:10.1086/302159. PMC 1377635. PMID 9837816.
- Guldberg P, thor Straten P, Ahrenkiel V, et al. (1999). "Somatic mutation of the Peutz-Jeghers syndrome gene, LKB1/STK11, in malignant melanoma". Oncogene. 18 (9): 1777–80. doi:10.1038/sj.onc.1202486. PMID 10208439.
- Su GH, Hruban RH, Bansal RK, et al. (1999). "Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers". Am. J. Pathol. 154 (6): 1835–40. doi:10.1016/S0002-9440(10)65440-5. PMC 1866632. PMID 10362809.
- Westerman AM, Entius MM, Boor PP, et al. (1999). "Novel mutations in the LKB1/STK11 gene in Dutch Peutz-Jeghers families". Hum. Mutat. 13 (6): 476–81. doi:10.1002/(SICI)1098-1004(1999)13:6<476::AID-HUMU7>3.0.CO;2-2. PMID 10408777.
- Scanlan MJ, Gordan JD, Williamson B, et al. (1999). "Antigens recognized by autologous antibody in patients with renal-cell carcinoma". Int. J. Cancer. 83 (4): 456–64. doi:10.1002/(SICI)1097-0215(19991112)83:4<456::AID-IJC4>3.0.CO;2-5. PMID 10508479.
- Collins SP, Reoma JL, Gamm DM, Uhler MD (2000). "LKB1, a novel serine/threonine protein kinase and potential tumour suppressor, is phosphorylated by cAMP-dependent protein kinase (PKA) and prenylated in vivo". Biochem. J. 345 (3): 673–80. doi:10.1042/0264-6021:3450673. PMC 1220803. PMID 10642527.
- Sapkota GP, Kieloch A, Lizcano JM, et al. (2001). "Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell vrowth". J. Biol. Chem. 276 (22): 19469–82. doi:10.1074/jbc.M009953200. PMID 11297520.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Karuman P, Gozani O, Odze RD, et al. (2001). "The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death". Mol. Cell. 7 (6): 1307–19. doi:10.1016/S1097-2765(01)00258-1. PMID 11430832.
- Marignani PA, Kanai F, Carpenter CL (2001). "LKB1 associates with Brg1 and is necessary for Brg1-induced growth arrest". J. Biol. Chem. 276 (35): 32415–8. doi:10.1074/jbc.C100207200. PMID 11445556.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Carretero J, Medina PP, Pio R, Montuenga LM, Sanchez-Cespedes M (2004). "Novel and natural knockout lung cancer cell lines for the LKB1/STK11 tumor suppressor gene". Cancer Res. 23 (22): 4037–40. doi:10.1038/sj.onc.1207502. PMID 15021901.
- Abed AA, Günther K, Kraus C, et al. (2002). "Mutation screening at the RNA level of the STK11/LKB1 gene in Peutz-Jeghers syndrome reveals complex splicing abnormalities and a novel mRNA isoform (STK11 c.597(insertion mark)598insIVS4)". Hum. Mutat. 18 (5): 397–410. doi:10.1002/humu.1211. PMID 11668633.
- Sato N, Rosty C, Jansen M, et al. (2001). "STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas". Am. J. Pathol. 159 (6): 2017–22. doi:10.1016/S0002-9440(10)63053-2. PMC 1850608. PMID 11733352.
External links
This article incorporates text from the United States National Library of Medicine, which is in the public domain.