Graduated cylinder: Difference between revisions
m Reverted edits by 198.209.35.251 (talk) (HG) (3.1.21) |
|||
| Line 4: | Line 4: | ||
== Materials & Structure == |
== Materials & Structure == |
||
[[File:Graduated cylinder illustration.png|thumb|294x294px|If the reading is done and the value calculated is set to be 36.5 mL. The more precise value equates to 36.5 <math>\pm</math> 0.1; 36.4 or 36.6 mL.]] |
[[File:Graduated cylinder illustration.png|thumb|294x294px|If the reading is done and the value calculated is set to be 36.5 mL. The more precise value equates to 36.5 <math>\pm</math> 0.1; 36.4 or 36.6 mL.]] |
||
Large graduated cylinders are usually made up of [[polypropylene]] for its excellent chemical resistance or [[polymethylpentene]] for its transparency, making them lighter and less [[Brittle|fragile]] than [[glass]]. [[Polypropylene]] (PP) is easy to repeatedly [[autoclave]]; however, autoclaving in excess of about {{convert|121|°C}} (depending on the chemical formulation: typical commercial grade [[polypropylene]] melts in excess of {{convert|177|°C}}), can warp or damage polypropylene graduated cylinders, affecting accuracy.<ref>{{Cite web|title = Graduated Cylinders, Plastic - SPI Supplies|url = http://www.2spi.com/catalog/ |
Large graduated cylinders are usually made up of [[polypropylene]] for its excellent chemical resistance or [[polymethylpentene]] for its transparency, making them lighter and less [[Brittle|fragile]] than [[glass]]. [[Polypropylene]] (PP) is easy to repeatedly [[autoclave]]; however, autoclaving in excess of about {{convert|121|°C}} (depending on the chemical formulation: typical commercial grade [[polypropylene]] melts in excess of {{convert|177|°C}}), can warp or damage polypropylene graduated cylinders, affecting accuracy.<ref>{{Cite web|title = Graduated Cylinders, Plastic - SPI Supplies|url = http://www.2spi.com/catalog/p HILLARY IS THE DEVIL |
||
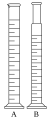
I H N traditional graduated cylinder (A in the image) is usually narrow and tall so as to increase the accuracy and precision of volume measurement; it has a plastic or glass bottom and a "spout" for easy pouring of the measured liquid. An additional version is wide and low. |
I H N traditional graduated cylinder (A in the image) is usually narrow and tall so as to increase the accuracy and precision of volume measurement; it has a plastic or glass bottom and a "spout" for easy pouring of the measured liquid. An additional version is wide and low. |
||
Revision as of 14:29, 14 September 2016

A graduated cylinder, measuring cylinder or mixing cylinder is a common piece of laboratory equipment used to measure the volume of a liquid. It has a narrow cylindrical shape. Each marked line on the graduated cylinder represents the amount of liquid that has been measured.
Materials & Structure

Large graduated cylinders are usually made up of polypropylene for its excellent chemical resistance or polymethylpentene for its transparency, making them lighter and less fragile than glass. Polypropylene (PP) is easy to repeatedly autoclave; however, autoclaving in excess of about 121 °C (250 °F) (depending on the chemical formulation: typical commercial grade polypropylene melts in excess of 177 °C (351 °F)), can warp or damage polypropylene graduated cylinders, affecting accuracy.Cite error: A <ref> tag is missing the closing </ref> (see the help page). With this kind of cylinder, the metered liquid does not pour directly, but is often removed using a cannula. A graduated cylinder is meant to be read with the surface of the liquid at eye level, where the center of the meniscus shows the measurement line. Typical capacities of graduated cylinders are from 10 ml to 1000 ml.
Common uses
Graduated cylinders are often used to measure the volume of a liquid. Graduated cylinders are generally more accurate and precise than laboratory flasks and beakers, but they should not be used to perform volumetric analysis;[1] volumetric glassware, such as a volumetric flask or volumetric pipette, should be used, as it is even more accurate and precise. Graduated cylinders are sometimes used to measure the volume of a solid indirectly by measuring the displacement of a liquid.
Scales & Accuracy
For accuracy the volume on graduated cylinders is depicted on scales with 3 significant digits: 100ml cylinders have 1ml grading divisions while 10ml cylinders have 0.1 ml grading divisions.
Two classes of accuracy exist for graduated cylinders. Class A has the double accuracy of class B.[2] Cylinders can have single or double scales. Single scales allow to read the volume from top to bottom (filling volume) while double scale cylinders allow reading for filling and pouring (reverse scale).
Graduated cylinders are calibrated either “to contain” (indicated liquid volume inside the cylinder) and marked as "TC" or “to deliver” (indicated liquid volume poured out, accounting for liquid traces left in the cylinder) and marked “TD”.[3] Formerly the tolerances for “to deliver” and “to contain” cylinders are distinct; however now these are the same. Also, the international symbols “IN” and “EX” are more likely to be used instead of “TC” and “TD” respectively.[4]
Measurement

To read the volume accurately, the observation must be at an eye level and read at the bottom of a meniscus of the liquid level.[5] For example, in the picture on the bottom of the page, a 100ml graduated cylinder was used to measure the liquid. The cylinder contains 60ml liquid volume. The main reason as to why the reading of the volume is done via meniscus is due to the nature of the liquid in a closed surrounded space. By nature, liquid in the cylinder would be attracted to the wall around it through molecular forces. This forces the liquid surface to develop either a convex or concave shape, depending on the type of the liquid in the cylinder. Reading the liquid at the bottom part of a concave or the top part of the convex liquid is equivalent to reading the liquid at its meniscus.[6] From the picture, the level of the liquid will be read at the bottom of the meniscus, which is the concave. The most accurate of the reading that could be done here is reduced down to 1 ml due to the given means of measurement on the cylinder. From this, the derived error would be one tenth of the least figure. For instance, if the reading is done and the value calculated is set to be 36.5 mL. The error, give or take 0.1 mL, must be included too. Therefore, the more precise value equates to 36.5 0.1; 36.4 or 36.6 mL. Therefore, there are 3 significant figures can be read from the given graduated cylinder picture.[7] Another example, if the reading is done and the value calculated is set to be 40.0 mL. The precise value would be 40.0 0.1; 40.1 or 39.9 mL.[8]
Additional images
-
100ml graduated cylinder
-
Two graduated cylinders. I H N traditional graduated cylinder (A in the image), and mixing cylinders (B in the picture)
-
60ml volume measured by graduated cylinder
References
- ^ Pradyot Patnaik (2003). "Specifications for volumetric ware". Dean's Handbook of Analytical Chemistry, 2nd Edition. McGraw-Hill. ISBN 978-0071410601.
- ^ http://www.astm.org/Standards/E1272.htm
- ^ "Graduated Cylinders Information".
- ^ "Graduated Cylinders". sizes.com. Retrieved 2016-02-23.
- ^ "graduated cylinder" (PDF). ohlone.edu. Retrieved 2015-06-25.
- ^ "Volume Measurements with a Graduated Cylinder" (PDF). Retrieved 2016-02-04.
- ^ "Math Skills - Scientific Notation". www.chem.tamu.edu. Retrieved 2016-02-12.
- ^ Robinson, Michael; Robinson, Mike; Taylor, Mike (2002-01-01). Maths for Advanced Chemistry. Nelson Thornes. ISBN 9780748765829. Retrieved 15 March 2016.