Magnetic nanoparticles: Difference between revisions
m Dating maintenance tags: {{Primary}} |
Jvdmehrdad (talk | contribs) |
||
| Line 108: | Line 108: | ||
=== Biomedical imaging === |
=== Biomedical imaging === |
||
There are many applications for iron-oxide based nanoparticles in concert with [[magnetic resonance imaging]].<ref>{{cite journal | last1 = Colombo | first1 = M | display-authors = etal | year = 2012 | title = Biological Applications of Magnetic Nanoparticles | url = | journal = Chem Soc Rev | volume = 41 | issue = 11| pages = 4306–34 | doi=10.1039/c2cs15337h}}</ref> Magnetic CoPt nanoparticles are being used as an MRI contrast agent for transplanted [[neural stem cell]] detection.<ref>{{cite journal|last=Xiaoting Meng |author2=Hugh C. Seton |author3=Le T. Lu |author4=Ian A. Prior |author5=Nguyen T. K. Thanh |author6=Bing Song|journal=Nanoscale|year=2011|volume=3|pages=977–984|doi=10.1039/C0NR00846J|bibcode = 2011Nanos...3..977M|title=Magnetic CoPt nanoparticles as MRI contrast agent for transplanted neural stem cells detection|first1=Xiaoting|issue=3|pmid=21293831 }}</ref> |
There are many applications for iron-oxide based nanoparticles in concert with [[magnetic resonance imaging]].<ref>{{cite journal | last1 = Colombo | first1 = M | display-authors = etal | year = 2012 | title = Biological Applications of Magnetic Nanoparticles | url = | journal = Chem Soc Rev | volume = 41 | issue = 11| pages = 4306–34 | doi=10.1039/c2cs15337h}}</ref> Magnetic CoPt nanoparticles are being used as an MRI contrast agent for transplanted [[neural stem cell]] detection.<ref>{{cite journal|last=Xiaoting Meng |author2=Hugh C. Seton |author3=Le T. Lu |author4=Ian A. Prior |author5=Nguyen T. K. Thanh |author6=Bing Song|journal=Nanoscale|year=2011|volume=3|pages=977–984|doi=10.1039/C0NR00846J|bibcode = 2011Nanos...3..977M|title=Magnetic CoPt nanoparticles as MRI contrast agent for transplanted neural stem cells detection|first1=Xiaoting|issue=3|pmid=21293831 }}</ref> |
||
=== Cancer Therapy === |
|||
In magnetic fluid hyperthermia, nanoparticles of different types like Iron oxide, magnetite, maghemite or even gold are injected in tumor and then subjected under a high frequency magnetic field. These nanoparticles produce heat that can increase temperature up to 42 degree which can be useful for destroying cancerous cells.<ref>http://www.tandfonline.com/doi/abs/10.3109/02656736.2014.988661</ref><ref>http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4289522/</ref><ref>http://www.worldscientific.com/doi/abs/10.1142/S0219519415500888</ref> |
|||
=== Information storage === |
=== Information storage === |
||
Revision as of 01:15, 27 September 2016
Magnetic nanoparticles are a class of nanoparticle that can be manipulated using magnetic fields. Such particles commonly consist of two components, a magnetic material, often iron, nickel and cobalt, and a chemical component that has functionality. While nanoparticles are smaller than 1 micrometer in diameter (typically 5–500 nanometers), the larger microbeads are 0.5–500 micrometer in diameter. Magnetic nanoparticle clusters that are composed of a number of individual magnetic nanoparticles are known as magnetic nanobeads with a diameter of 50–200 nanometers.[1] Magnetic nanoparticle clusters are a basis for their further magnetic assembly into magnetic nanochains.[2] The magnetic nanoparticles have been the focus of much research recently because they possess attractive properties which could see potential use in catalysis including nanomaterial-based catalysts,[3] biomedicine [4] and tissue specific targeting,[5] magnetically tunable colloidal photonic crystals,[6] microfluidics,[7] magnetic resonance imaging,[8] magnetic particle imaging,[9] data storage,[10][11] environmental remediation,[12] nanofluids,[13] and optical filters,[14] defect sensor [15] and cation sensors.[16]
Properties
The physical and chemical properties of magnetic nanoparticles largely depend on the synthesis method and chemical structure. In most cases, the particles range from 1 to 100 nm in size and may display superparamagnetism.[17]
Types of magnetic nanoparticles
Oxides: ferrites
Ferrite nanoparticles or iron oxide nanoparticles (iron oxides in crystal structure of maghemite or magnetite) are the most explored magnetic nanoparticles up to date. Once the ferrite particles become smaller than 128 nm[18] they become superparamagnetic which prevents self agglomeration since they exhibit their magnetic behavior only when an external magnetic field is applied. The magnetic moment of ferrite nanoparticles can be greatly increased by controlled clustering of a number of individual superparamagnetic nanoparticles into superparamagnetic nanoparticle clusters, namely magnetic nanobeads.[1] With the external magnetic field switched off, the remanence falls back to zero. Just like non-magnetic oxide nanoparticles, the surface of ferrite nanoparticles is often modified by surfactants, silica,[1] silicones or phosphoric acid derivatives to increase their stability in solution.[19]
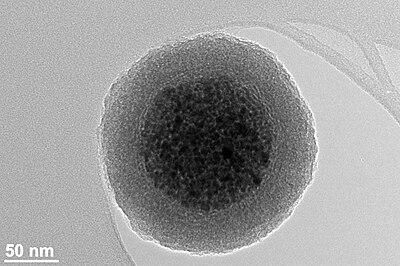
Ferrites with a shell
The surface of a maghemite or magnetite magnetic nanoparticle is relatively inert and does not usually allow strong covalent bonds with functionalization molecules. However, the reactivity of the magnetic nanoparticles can be improved by coating a layer of silica onto their surface.[21] The silica shell can be easily modified with various surface functional groups via covalent bonds between organo-silane molecules and silica shell.[22] In addition, some fluorescent dye molecules can be covalently bonded to the functionalized silica shell.[23]
Ferrite nanoparticle clusters with narrow size distribution consisting of superparamagnetic oxide nanoparticles (~ 80 maghemite superparamagnetic nanoparticles per bead) coated with a silica shell have several advantages over metallic nanoparticles:[1]
- Higher chemical stability (crucial for biomedical applications)
- Narrow size distribution (crucial for biomedical applications)
- Higher colloidal stability since they do not magnetically agglomerate
- Magnetic moment can be tuned with the nanoparticle cluster size
- Retained superparamagnetic properties (independent of the nanoparticle cluster size)
- Silica surface enables straightforward covalent functionalization
Metallic
Metallic nanoparticles may be beneficial for some technical applications due to their higher magnetic moment whereas oxides (maghemite, magnetite) would be beneficial for biomedical applications. This also implies that for the same moment, metallic nanoparticles can be made smaller than their oxide counterparts. On the other hand, metallic nanoparticles have the great disadvantage of being pyrophoric and reactive to oxidizing agents to various degrees. This makes their handling difficult and enables unwanted side reactions which makes them less appropriate for biomedical applications. Colloid formation for metallic particles is also much more challenging.
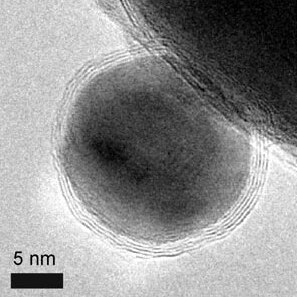
Metallic with a shell
The metallic core of magnetic nanoparticles may be passivated by gentle oxidation, surfactants, polymers and precious metals.[17] In an oxygen environment, Co nanoparticles form an anti-ferromagnetic CoO layer on the surface of the Co nanoparticle. Recently, work has explored the synthesis and exchange bias effect in these Co core CoO shell nanoparticles with a gold outer shell.[25] Nanoparticles with a magnetic core consisting either of elementary Iron or Cobalt with a nonreactive shell made of graphene have been synthesized recently.[26] The advantages compared to ferrite or elemental nanoparticles are:
- Higher magnetization
- Higher stability in acidic and basic solution as well as organic solvents
- Chemistry[24] on the graphene surface via methods already known for carbon nanotubes
Synthesis
Several methods exist for preparing magnetic nanoparticle.
Co-precipitation
Co-precipitation is a facile and convenient way to synthesize iron oxides (either Fe3O4 or γ-Fe2O3) from aqueous Fe2+/Fe3+ salt solutions by the addition of a base under inert atmosphere at room temperature or at elevated temperature. The size, shape, and composition of the magnetic nanoparticles very much depends on the type of salts used (e.g.chlorides, sulfates, nitrates), the Fe2+/Fe3+ ratio, the reaction temperature, the pH value and ionic strength of the media,[17] and the mixing rate with the base solution used to provoke the precipitation.[27] The co-precipitation approach has been used extensively to produce ferrite nanoparticles of controlled sizes and magnetic properties.[28][29][30][31] A variety of experimental arrangements have been reported to facilitate continuous and large–scale co–precipitation of magnetic particles by rapid mixing.[32][33] Recently, the growth rate of the magnetic nanoparticles was measured in real-time during the precipitation of magnetite nanoparticles by an integrated AC magnetic susceptometer within the mixing zone of the reactants.[34]
Thermal decomposition
Magnetic nanocrystals with smaller size can essentially be synthesized through the thermal decomposition of alkaline organometallic compounds in high-boiling organic solvents containing stabilizing surfactants.[17]
Microemulsion
Using the microemulsion technique, metallic cobalt, cobalt/platinum alloys, and gold-coated cobalt/platinum nanoparticles have been synthesized in reverse micelles of cetyltrimethlyammonium bromide, using 1-butanol as the cosurfactant and octane as the oil phase.,[17][35]
Flame spray synthesis
Using flame spray pyrolysis [26][36] and varying the reaction conditions, oxides, metal or carbon coated nanoparticles are produced at a rate of > 30 g/h .
Potential applications
A wide variety of potential applications have been envisaged. Since magnetic nanoparticles are expensive to produce, there is interest in their recycling or for highly specialized applications. They are only used in scientific research. An industrial use has yet to be established.
The potential and versatility of magnetic chemistry arises from the fast and easy separation of the magnetic nanoparticles, eliminating tedious and costly separation processes usually applied in chemistry. Furthermore the magnetic nanoparticles can be guided via a magnetic field to the desired location which could, for example, enable pinpoint precision in fighting cancer.
Medical diagnostics and treatments
Magnetic nanoparticles have been examined for use in an experimental cancer treatment called magnetic hyperthermia [37] in which the fact that nanoparticles heat when they are placed in an alternative magnetic field is used.
Affinity ligands such as epidermal growth factor (EGF), folic acid, aptamers, lectins etc. can be attached to the magnetic nanoparticle surface with the use of various chemistries. This enables targeting of magnetic nanoparticles to specific tissues or cells.[38] This strategy is used in cancer research to target and treat tumors in combination with magnetic hyperthermia or nanoparticle-delivered cancer drugs.
Another potential treatment of cancer includes attaching magnetic nanoparticles to free-floating cancer cells, allowing them to be captured and carried out of the body. The treatment has been tested in the laboratory on mice and will be looked at in survival studies.[39][40]
Magnetic nanoparticles can be used for the detection of cancer. Blood can be inserted onto a microfluidic chip with magnetic nanoparticles in it. These magnetic nanoparticles are trapped inside due to an externally applied magnetic field as the blood is free to flow through. The magnetic nanoparticles are coated with antibodies targeting cancer cells or proteins. The magnetic nanoparticles can be recovered and the attached cancer-associated molecules can be assayed to test for their existence.
Magnetic nanoparticles can be conjugated with carbohydrates and used for detection of bacteria. Iron oxide particles have been used for the detection of Gram negative bacteria like Escherichia coli and for detection of Gram positive bacteria like Streptococcus suis[41][42]
Magnetic immunoassay
Magnetic immunoassay[43] (MIA) is a novel type of diagnostic immunoassay utilizing magnetic nanobeads as labels in lieu of conventional, enzymes, radioisotopes or fluorescent moieties. This assay involves the specific binding of an antibody to its antigen, where a magnetic label is conjugated to one element of the pair. The presence of magnetic nanobeads is then detected by a magnetic reader (magnetometer) which measures the magnetic field change induced by the beads. The signal measured by the magnetometer is proportional to the analyte (virus, toxin, bacteria, cardiac marker,etc.) quantity in the initial sample.
Waste water treatment
Thanks to the easy separation by applying a magnetic field and the very large surface to volume ratio, magnetic nanoparticles have a potential for treatment of contaminated water.[44] In this method, attachment of EDTA-like chelators to carbon coated metal nanomagnets results in a magnetic reagent for the rapid removal of heavy metals from solutions or contaminated water by three orders of magnitude to concentrations as low as micrograms per Litre. Magnetic nanobeads or nanoparticle clusters composed of FDA-approved oxide superparamagnetic nanoparticles (e.g. maghemite, magnetite) hold much potential for waste water treatment since they express excellent biocompatibility which concerning the environmental impacts of the material is an advantage compared to metallic nanoparticles.
Supported enzymes and peptides
Enzymes, proteins, and other biologically and chemically active substances have been immobilized on magnetic nanoparticles.[45] They are of interest as possible supports for solid phase synthesis.[46]
This technology is potentially relevant to cellular labelling/cell separation, detoxification of biological fluids, tissue repair, drug delivery, magnetic resonance imaging, hyperthermia and magnetofection.[47]
Catalyst support
Magnetic nanoparticles are of potential use as a catalyst or catalyst supports.[48] In chemistry, a catalyst support is the material, usually a solid with a high surface area, to which a catalyst is affixed. The reactivity of heterogeneous catalysts occurs at the surface atoms. Consequently, great effort is made to maximize the surface area of a catalyst by distributing it over the support. The support may be inert or participate in the catalytic reactions. Typical supports include various kinds of carbon, alumina, and silica. Immobilizing the catalytic center on top of nanoparticles with a large surface to volume ratio addresses this problem. In the case of magnetic nanoparticles it adds the property of facile a separation. An early example involved a rhodium catalysis attached to magnetic nanoparticles .[49]

In another example, the stable radical TEMPO was attached to the graphene-coated cobalt nanoparticles via a diazonium reaction. The resulting catalyst was then used for the chemoselective oxidation of primary and secondary alcohols.[50]
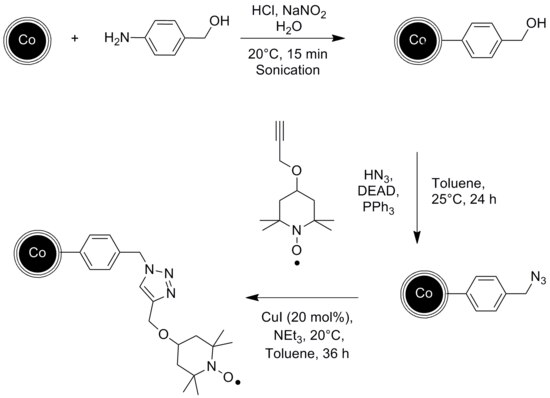
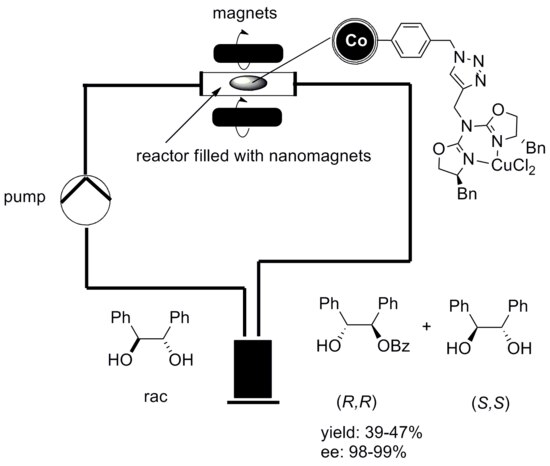
The catalytic reaction can be conducted in a continuous flow reactor instead of a batch reactor with no remains of the catalyst in the end product. Graphene coated cobalt nanoparticles have been used for that experiment since they exhibit a higher magnetization than Ferrite nanoparticles, which is essential for a fast and clean separation via external magnetic field.[51]
Biomedical imaging
There are many applications for iron-oxide based nanoparticles in concert with magnetic resonance imaging.[52] Magnetic CoPt nanoparticles are being used as an MRI contrast agent for transplanted neural stem cell detection.[53]
Cancer Therapy
In magnetic fluid hyperthermia, nanoparticles of different types like Iron oxide, magnetite, maghemite or even gold are injected in tumor and then subjected under a high frequency magnetic field. These nanoparticles produce heat that can increase temperature up to 42 degree which can be useful for destroying cancerous cells.[54][55][56]
Information storage
A promising candidate for high-density storage is the face-centered tetragonal phase FePt alloy. Grain sizes can be as small as 3 nanometers. If it's possible to modify the MNPs at this small scale, the information density that can be achieved with this media could easily surpass 1 Terabyte per square inch.[11]
Genetic engineering
Magnetic nanoparticles can be used for a variety of genetics applications. One application is the rapid isolation of mRNA. In one application, the magnetic bead is attached to a poly T tail. When mixed with mRNA, the poly A tail of the mRNA will attach to the bead's poly T tail and the isolation takes place simply by placing a magnet on the side of the tube and pouring out the liquid. Magnetic beads have also been used in plasmid assembly. Rapid genetic circuit construction has been achieved by the sequential addition of genes onto a growing genetic chain, using nanobeads as an anchor. This method has been shown to be much faster than previous methods, taking less than an hour to create functional multi-gene constructs in vitro.[57]
See also
References
- ^ a b c d Tadic, Marin; Kralj, Slavko; Jagodic, Marko; Hanzel, Darko; Makovec, Darko (December 2014). "Magnetic properties of novel superparamagnetic iron oxide nanoclusters and their peculiarity under annealing treatment". Applied Surface Science. 322: 255–264. Bibcode:2014ApSS..322..255T. doi:10.1016/j.apsusc.2014.09.181.
- ^ Kralj, Slavko; Makovec, Darko (27 October 2015). "Magnetic Assembly of Superparamagnetic Iron Oxide Nanoparticle Clusters into Nanochains and Nanobundles". ACS Nano. 9 (10): 9700–9707. doi:10.1021/acsnano.5b02328.
- ^ A.-H. Lu; W. Schmidt; N. Matoussevitch; H. Bönnemann; B. Spliethoff; B. Tesche; E. Bill; W. Kiefer; F. Schüth (August 2004). "Nanoengineering of a Magnetically Separable Hydrogenation Catalyst". Angewandte Chemie International Edition. 43 (33): 4303–4306. doi:10.1002/anie.200454222. PMID 15368378.
- ^ A. K. Gupta; M. Gupta (June 2005). "Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications". Biomaterials. 26 (18): 3995–4021. doi:10.1016/j.biomaterials.2004.10.012. PMID 15626447.
- ^ Ramaswamy, B; Kulkarni, SD; Villar, PS; Smith, RS; Eberly, C; Araneda, RC; Depireux, DA; Shapiro, B (24 June 2015). "Movement of magnetic nanoparticles in brain tissue: mechanisms and safety". Nanomedicine: Nanotechnology, Biology and Medicine. 11: 1821–9. doi:10.1016/j.nano.2015.06.003. PMID 26115639.
- ^ He, Le; Wang, Mingsheng; Ge, Jianping; Yin, Yadong (18 September 2012). "Magnetic Assembly Route to Colloidal Responsive Photonic Nanostructures". Accounts of Chemical Research. 45 (9): 1431–1440. doi:10.1021/ar200276t. PMID 22578015.
- ^ Kavre, Ivna; Kostevc, Gregor; Kralj, Slavko; Vilfan, Andrej; Babič, Dušan (13 August 2014). "Fabrication of magneto-responsive microgears based on magnetic nanoparticle embedded PDMS". RSC Advances. 4 (72): 38316–38322. doi:10.1039/C4RA05602G.
- ^ S. Mornet; S. Vasseur; F. Grasset; P. Verveka; G. Goglio; A. Demourgues; J. Portier; E. Pollert; E. Duguet (2006). Prog. Solid State Chem. 34: 237.
{{cite journal}}: Missing or empty|title=(help) - ^ B. Gleich; J. Weizenecker (2005). "Tomographic imaging using the nonlinear response of magnetic particles". Nature. 435 (7046): 1214–1217. Bibcode:2005Natur.435.1214G. doi:10.1038/nature03808. PMID 15988521.
- ^ Hyeon, Taeghwan (3 April 2003). "Chemical synthesis of magnetic nanoparticles". Chemical Communications (8): 927–934. doi:10.1039/B207789B.
- ^ a b Natalie A. Frey and Shouheng Sun Magnetic Nanoparticle for Information Storage Applications
- ^ Elliott, Daniel W.; Zhang, Wei-xian (December 2001). "Field Assessment of Nanoscale Bimetallic Particles for Groundwater Treatment". Environmental Science & Technology. 35 (24): 4922–4926. Bibcode:2001EnST...35.4922E. doi:10.1021/es0108584.
- ^ J. Philip; Shima.P.D. B. Raj (2006). "Nanofluid with tunable thermal properties". Applied Physics Letters. 92: 043108. Bibcode:2008ApPhL..92d3108P. doi:10.1063/1.2838304.
- ^ J.Philip, T.J.Kumar, P.Kalyanasundaram, B.Raj (2003). "Tunable Optical Filter". Measurement Science & Technology. 14: 1289–1294. Bibcode:2003MeScT..14.1289P. doi:10.1088/0957-0233/14/8/314.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mahendran, V. "Nanofluid based opticalsensor for rapid visual inspection of defects in ferromagnetic materials". Appl. Phys. Lett. 100: 073104. Bibcode:2012ApPhL.100g3104M. doi:10.1063/1.3684969.
- ^ Philip, V. Mahendran; Felicia, Leona J. (2013). "A Simple, In-Expensive and Ultrasensitive Magnetic Nanofluid Based Sensor for Detection of Cations, Ethanol and Ammonia". J. Nanofluids. 2: 112–119. doi:10.1166/jon.2013.1050.
- ^ a b c d e A.-H. Lu, E. L. Salabas, and F. Schüth (2007). "Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application". Angew. Chem. Int. Ed. 46 (8): 1222–1244. doi:10.1002/anie.200602866.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ An-Hui Lu, An-Hui; E. L. Salabas; Ferdi Schüth (2007). "Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application". Angew. Chem. Int. Ed. 46 (8): 1222–1244. doi:10.1002/anie.200602866.
- ^ Kim, DK, G.; Mikhaylova, M; et al. (2003). "Anchoring of Phosphonate and Phosphinate Coupling Molecules on Titania Particles". Chemistry of Materials. 15 (8): 1617–1627. doi:10.1021/cm001253u.
- ^ http://nanos-sci.com/technology.html Properties and use of magnetic nanoparticle clusters (magnetic nanobeads)
- ^ Kralj, Slavko; Makovec, Darko; Čampelj, Stanislav; Drofenik, Miha (July 2010). "Producing ultra-thin silica coatings on iron-oxide nanoparticles to improve their surface reactivity". Journal of Magnetism and Magnetic Materials. 322 (13): 1847–1853. Bibcode:2010JMMM..322.1847K. doi:10.1016/j.jmmm.2009.12.038.
- ^ Kralj, Slavko; Drofenik, Miha; Makovec, Darko (16 December 2010). "Controlled surface functionalization of silica-coated magnetic nanoparticles with terminal amino and carboxyl groups". Journal of Nanoparticle Research. 13 (7): 2829–2841. doi:10.1007/s11051-010-0171-4.
- ^ Kralj, Slavko; Rojnik, Matija; Romih, Rok; Jagodič, Marko; Kos, Janko; Makovec, Darko (7 September 2012). "Effect of surface charge on the cellular uptake of fluorescent magnetic nanoparticles". Journal of Nanoparticle Research. 14 (10). doi:10.1007/s11051-012-1151-7.
- ^ a b R.N. Grass, Robert N.; E.K. Athanassiou; W.J. Stark (2007). "Covalently Functionalized Cobalt Nanoparticles as a Platform for Magnetic Separations in Organic Synthesis". Angew. Chem. Int. Ed. 46 (26): 4909–12. doi:10.1002/anie.200700613.
- ^ Johnson, Stephanie H.; C.L. Johnson; S.J. May; S. Hirsch; M.W. Cole; J.E. Spanier (2010). "Co@CoO@Au core-multi-shell nanocrystals". Journal of Materials Chemistry. 20 (3): 439–443. doi:10.1039/b919610b.
- ^ a b R. N. Grass, Robert N.; W. J. Stark (2006). "Gas phase synthesis of fcc-cobalt nanoparticles". J. Mater. Chem. 16 (16): 1825. doi:10.1039/B601013J.
- ^ Fang, Mei; Ström, Valter; Olsson, Richard T.; Belova, Lyubov; Rao, K. V. "Rapid mixing: A route to synthesize magnetite nanoparticles with high moment". Appl. Phys. Lett. 99: 222501. Bibcode:2011ApPhL..99v2501F. doi:10.1063/1.3662965.
- ^ G.Gnanaprakash, S.Ayyappan, T.Jayakumar, John Philip & Baldev Raj (2006). "A simple method to produce magnetic nanoparticles with enhanced alpha to gamma-Fe2O3 phase transition temperature". Nanotechnology. 17: 5851–5857. Bibcode:2006Nanot..17.5851G. doi:10.1088/0957-4484/17/23/023.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ G. Gnanaprakash; John Philip; T. Jayakumar; Baldev Raj (2007). "Effect of Digestion Time and Alkali Addition Rate on the Physical Properties of Magnetite Nanoparticles". J. Phys. Chem. B. 111: 7978–7986. doi:10.1021/jp071299b.
- ^ S.Ayyappan, John Philip; Baldev Raj (2009). "Solvent polarity effect on physical properties of CoFe2O3 nanoparticles". J. Phys. Chem. C. 113: 590–596. doi:10.1021/jp8083875.
{{cite journal}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ S. Ayyappan, S. Mahadevan, P. Chandramohan, M. P.Srinivasan, John Philip & Baldev Raj (2010). "Influence of Co2 Ion Concentration on the Size, Magnetic Properties, and Purity of CoFe2O4 Spinel Ferrite Nanoparticles". J. Phys. Chem. C. 114: 6334–6341. doi:10.1021/jp911966p.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fun Chin, Suk; Iyer, K. Swaminathan; Raston, Colin L.; Saunders, Martin (2008). "Size Selective Synthesis of Superparamagnetic Nanoparticles in Thin Fluids under Continuous Flow Conditions". Adv. Funct. Mater. 18: 922–927. doi:10.1002/adfm.200701101.
- ^ Nigel Smith, Colin L. Raston, Martin Saunders, Robert Woodward; http://www.nsti.org/publications/Nanotech/2006/pdf/567.pdf
- ^ Ström, Valter; Olsson, Richard T.; Rao, K. V. (2010). J. Mater. Chem. 20: 4168–4175.
{{cite journal}}: Missing or empty|title=(help) - ^ S S.Rana, J. Philip, B.Raj (2010). "Micelle based synthesis of Cobalt Ferrite nanoparticles and its characterization using Fourier Transform Infrared Transmission Spectrometry and Thermogravimetry". Materials Chemistry and Physics. 124: 264–269. doi:10.1016/j.matchemphys.2010.06.029.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ E. K. Athanassiou, Evagelos K.; R. N. Grass; W. J. Stark (2010). "Chemical Aerosol Engineering as a Novel Tool for Material Science: From Oxides to Salt and Metal Nanoparticles". Aerosol. Sci. Tech. 44 (2): 161–72. doi:10.1080/02786820903449665.
- ^ Rabias, I.; et al. "Rapid magnetic heating treatment by highly charged maghemite nanoparticles on Wistar rats exocranial glioma tumors at microliter volume". Biomicrofluidics. 4: 024111. doi:10.1063/1.3449089.
- ^ Kralj, Slavko; Rojnik, Matija; Kos, Janko; Makovec, Darko (26 April 2013). "Targeting EGFR-overexpressed A431 cells with EGF-labeled silica-coated magnetic nanoparticles". Journal of Nanoparticle Research. 15 (5). doi:10.1007/s11051-013-1666-6.
- ^ Scarberry KE, Dickerson EB, McDonald JF, Zhang ZJ (2008). "Magnetic Nanoparticle-Peptide Conjugates for in Vitro and in Vivo Targeting and Extraction of Cancer Cells". Journal of the American Chemical Society. 130 (31): 10258–62. doi:10.1021/ja801969b. PMID 18611005.
- ^ Using Magnetic Nanoparticles to Combat Cancer Newswise, Retrieved on July 17, 2008.
- ^ Parera Pera N; Kouki A.; Finne J.; Pieters R. J. (2010). "Detection of pathogenic Streptococcus suis bacteria using magnetic glycoparticles". Organic & Biomolecular Chemi. 8 (10): 2425–2429. doi:10.1039/C000819B.
- ^ Highlights in Chemical Biology. Rsc.org (2007-06-13). Retrieved on 2011-10-07.
- ^ Magnetic immunoassays: A new paradigm in POCT IVDt, July/August 2008.
- ^ F.M. Koehler, Fabian M.; M. Rossier; M. Waelle; E.K. Athanassiou; L.K. Limbach; R.N. Grass; D. Günther; W.J. Stark (2009). "Magnetic EDTA: Coupling heavy metal chelators to metal nanomagnets for rapid removal of cadmium, lead and copper from contaminated water". Chem. Commun. 32 (32): 4862–4. doi:10.1039/B909447D.
- ^ Huang-Hao Yang, Huang-Hao; Shu-Qiong Zhang; Xiao-Lan Chen; Zhi-Xia Zhuang; Jin-Gou Xu; Xiao-Ru Wang (2004). "Magnetite-Containing Spherical Silica Nanoparticles for Biocatalysis and Bioseparations". Analytical Chemistry. 76 (5): 1316–1321. doi:10.1021/ac034920m. PMID 14987087.
- ^ K.Norén, Katarina; M. Kempe (2009). "Multilayered Magnetic Nanoparticles as a Support in Solid-Phase Peptide Synthesis". International Journal of Peptide Research and Therapeutics. 15 (4): 287–292. doi:10.1007/s10989-009-9190-3.
- ^ Gupta AK, Ajay Kumar; Gupta M (2005). "Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications". Biomaterials. 26 (18): 3995–4021. doi:10.1016/j.biomaterials.2004.10.012. PMID 15626447.
- ^ A. Schätz, Alexander; O. Reiser; W.J. Stark (2010). "Nanoparticles as Semi-Heterogeneous Catalyst Supports". Chem. Eur. J. 16 (30): 8950–67. doi:10.1002/chem.200903462.
- ^ Tae-Jong Yoon, Tae-Jong; Woo Lee; Yoon-Seuk Oh; Jin-Kyu Lee (2003). "Magnetic nanoparticles as a catalyst vehicle for simple and easy recyclingElectronic supplementary information (ESI) available: XRD and FT-IR data, as well as the detailed experimental conditions for the catalytic hydroformylation reactions. See http://www.rsc.org/suppdata/nj/b2/b209391j/". New Journal of Chemistry. 27 (2): 227.229. doi:10.1039/B209391J.
{{cite journal}}: External link in|title= - ^ A. Schätz, Alexander; R. N. Grass; W. J. Stark; O. Reiser (2008). "TEMPO Supported on Magnetic C/Co-Nanoparticles: A Highly Active and Recyclable Organocatalyst". Chemistry: A European Journal. 14 (27): 8262–8266. doi:10.1002/chem.200801001.
- ^ A. Schätz, Alexander; R. N. Grass; Q. Kainz; W. J. Stark; O. Reiser (2010). "Cu(II)−Azabis(oxazoline) Complexes Immobilized on Magnetic Co/C Nanoparticles: Kinetic Resolution of 1,2-Diphenylethane-1,2-diol under Batch and Continuous-Flow Conditions". Chemistry of Materials. 22 (2): 305–310. doi:10.1021/cm9019099.
- ^ Colombo, M; et al. (2012). "Biological Applications of Magnetic Nanoparticles". Chem Soc Rev. 41 (11): 4306–34. doi:10.1039/c2cs15337h.
- ^ Xiaoting Meng, Xiaoting; Hugh C. Seton; Le T. Lu; Ian A. Prior; Nguyen T. K. Thanh; Bing Song (2011). "Magnetic CoPt nanoparticles as MRI contrast agent for transplanted neural stem cells detection". Nanoscale. 3 (3): 977–984. Bibcode:2011Nanos...3..977M. doi:10.1039/C0NR00846J. PMID 21293831.
- ^ http://www.tandfonline.com/doi/abs/10.3109/02656736.2014.988661
- ^ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4289522/
- ^ http://www.worldscientific.com/doi/abs/10.1142/S0219519415500888
- ^ A Elaissari, J Chatterjee, M Hamoudeh and H Fessi (2010). "Chapter 14. Advances in the Preparation and Biomedical Applications of Magnetic Colloids". In Roque Hidalgo-Ålvarez (ed.). Structure and Functional Properties of Colloidal Systems. CRC Press. pp. 315–337. doi:10.1201/9781420084474-c14. ISBN 978-1-4200-8447-4.
{{cite book}}: CS1 maint: multiple names: authors list (link)
External links
- FML – Functional Materials Laboratory of the ETH Zürich
- Properties and use of magnetic nanoparticle clusters (magnetic nanobeads)
- Magnetic nanoparticles target human cancer cells
- Magnetic Nanoparticles Remove Ovarian Cancer Cells from the Abdominal Cavity
- Wiedwald, U. and Ziemann, P. (Ed.): Properties and applications of magnetic nanoparticles, Thematic Series in the Open Access Beilstein Journal of Nanotechnology.
