Syncytin-1: Difference between revisions
Jollyclause (talk | contribs) m rearranged text. Also to clear up nomenclature syncytin-1 is the protein name, ERVWe1 is the gene name (stands for Endogenous Retrovirus W family env member 1). ERVW1 is the provirus name (endogenous retrovirus W family member 1) |
Jollyclause (talk | contribs) m rearranged text |
||
| Line 7: | Line 7: | ||
Syncytin-1 is one of two known syncytin proteins expressed in humans and one of many syncytins captured and domesticated on multiple occasions over evolutionary time in diverse mammalian species<ref name="pmid23938756">{{cite journal|year=2013|title=Paleovirology of 'syncytins', retroviral env genes exapted for a role in placentation|journal=Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences|volume=368|issue=1626|pages=20120507|doi=10.1098/rstb.2012.0507|pmc=3758191|pmid=23938756|vauthors=Lavialle C, Cornelis G, Dupressoir A, Esnault C, Heidmann O, Vernochet C, Heidmann T}}</ref>. |
Syncytin-1 is one of two known syncytin proteins expressed in humans and one of many syncytins captured and domesticated on multiple occasions over evolutionary time in diverse mammalian species<ref name="pmid23938756">{{cite journal|year=2013|title=Paleovirology of 'syncytins', retroviral env genes exapted for a role in placentation|journal=Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences|volume=368|issue=1626|pages=20120507|doi=10.1098/rstb.2012.0507|pmc=3758191|pmid=23938756|vauthors=Lavialle C, Cornelis G, Dupressoir A, Esnault C, Heidmann O, Vernochet C, Heidmann T}}</ref>. |
||
| ⚫ | |||
| ⚫ | Synyctin-1 mediated [[trophoblast]] fusion is essential for normal [[placenta]]l development. The placenta is composed on two cell layers: [[cytotrophoblast]] and [[syncytiotrophoblast]] layer. Cytotrophoblasts are continually dividing, non-differentiated cells and syncytiotrophoblasts are fully differentiated, non dividing, fused cells Syncytin-1 expression on the surface of cytotrophoblasts and syncytiotrophoblasts mediate fusion. The syncytiotrophoblast layer is the necessary interface between the developing embryo and the maternal blood supply, allowing nutrient and waste exchange and blocking maternal immune cell invasion, preventing immune rejection of the foetus. Syncytiotrophoblasts are forced into [[senescence]] by fusion.<ref>{{cite journal | vauthors = Chuprin A, Gal H, Biron-Shental T, Biran A, Amiel A, Rozenblatt S, Krizhanovsky V | title = Cell fusion induced by ERVWE1 or measles virus causes cellular senescence | journal = Genes & Development | volume = 27 | issue = 21 | pages = 2356–66 | date = November 2013 | pmid = 24186980 | pmc = 3828521 | doi = 10.1101/gad.227512.113 }}</ref> Therefore, cytotrophoblast proliferation is necessary for growth and maintenance of the syncytiotrophoblast layer. Syncytin-1 expression in cytotrophoblasts promotes G1/S transition and proliferation thereby ensuring continual replenishment of the cytotrophoblast pool.<ref>{{cite journal | vauthors = Huang Q, Li J, Wang F, Oliver MT, Tipton T, Gao Y, Jiang SW | title = Syncytin-1 modulates placental trophoblast cell proliferation by promoting G1/S transition | journal = Cellular Signalling | volume = 25 | issue = 4 | pages = 1027–35 | date = April 2013 | pmid = 23333240 | pmc = 4644426 | doi = 10.1016/j.cellsig.2013.01.008 }}</ref> The name syncytin derives from its involvement in the formation of syncytium, the multinucleated syncytiotrophoblast protoplasm. There is another endogenous retroviral envelope protein expressed in the placenta from a different ERV family: syncytin-2 (of [[HERV-FRD]]). |
||
== Receptor == |
== Receptor == |
||
The syncytin-1 receptor is the Na-dependent amino acid transporter 2 ([[SLC1A5|ASCT2]] or [[SLC1A5]]).<ref>{{cite journal |
The syncytin-1 receptor is the Na-dependent amino acid transporter 2 ([[SLC1A5|ASCT2]] or [[SLC1A5]]).<ref>{{cite journal|date=July 2002|title=The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors|journal=Journal of Virology|volume=76|issue=13|pages=6442–52|doi=10.1128/JVI.76.13.6442-6452.2002|pmc=136247|pmid=12050356|vauthors=Lavillette D, Marin M, Ruggieri A, Mallet F, Cosset FL, Kabat D}}</ref><ref>{{cite journal|date=April 2000|title=An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor|journal=Journal of Virology|volume=74|issue=7|pages=3321–9|pmc=111833|pmid=10708449|vauthors=Blond JL, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel-Fernandes S, Mandrand B, Mallet F, Cosset FL}}</ref> This receptor places syncytin-1 in a large viral interference group called retroviral mammalian type D receptor (RDR) interference group.<ref name="pmid1691887">{{cite journal|date=May 1990|title=Receptor interference groups of 20 retroviruses plating on human cells|journal=Virology|volume=176|issue=1|pages=58–69|doi=10.1016/0042-6822(90)90230-O|pmid=1691887|vauthors=Sommerfelt MA, Weiss RA}}</ref> Syncytin-1 has been shown to interfere with viral infection ''in-vitro'' by RDR interference group member spleen necrosis virus.<ref>{{cite journal|date=April 2003|title=The envelope glycoprotein of human endogenous retrovirus HERV-W induces cellular resistance to spleen necrosis virus|journal=Archives of Virology|language=en|volume=148|issue=4|pages=659–75|doi=10.1007/s00705-002-0960-x|pmid=12664292|vauthors=Ponferrada VG, Mauck BS, Wooley DP}}</ref> Syncytin-1 can also recognize [[SLC1A4|ASCT1]] or [[SLC1A4]], but this receptor is not a receptor for the RDR interference group. Mutation studies of syncytin-1 and of ASCT2 have provided insight into potential receptor binding domains and determinants. A putative receptor binding domain was identified in syncytin-1 at residues 117-144.<ref>{{cite journal|date=2015-01-01|title=Human Endogenous Retroviruses as Pathogenic Factors in the Development of Schizophrenia|journal=Frontiers in Psychiatry|volume=6|pages=183|doi=10.3389/fpsyt.2015.00183|pmc=4707225|pmid=26793126|vauthors=Slokar G, Hasler G}}</ref> The amino acid sequence at this region is well conserved amongst RDR interference group members. The motif SDGGGX<sub>2</sub>DX<sub>2</sub>R is present in all RDR interference group members within this conserved region and may play an important role in binding. Preliminary evidence with syncytin-1 and spleen necrosis virus indicate this motif contains the ASCT2 binding determinants.<ref>{{cite journal|date=2015-01-01|title=Human Endogenous Retroviruses as Pathogenic Factors in the Development of Schizophrenia|journal=Frontiers in Psychiatry|volume=6|pages=183|doi=10.3389/fpsyt.2015.00183|pmc=4707225|pmid=26793126|vauthors=Slokar G, Hasler G}}</ref><ref>{{cite journal|date=July 1995|title=Mapping of receptor binding domains in the envelope protein of spleen necrosis virus|journal=Journal of Virology|volume=69|issue=7|pages=4339–46|pmc=189174|pmid=7769695|vauthors=Martinez I, Dornburg R}}</ref><ref>{{cite journal|date=September 1996|title=Mutational analysis of the envelope protein of spleen necrosis virus|url=http://www.ncbi.nlm.nih.gov/pmc/articles/PMC190624/|journal=Journal of Virology|volume=70|issue=9|pages=6036–43|pmc=190624|pmid=8709226|vauthors=Martinez I, Dornburg R}}</ref> |
||
The largest ectodomain of ASCT1 and ASCT2, extracellular loop 2 (ECL2), contains at its C-terminus a 21 residue hypervariable region between human, mouse, and hamster receptors. This region was shown to confer specificity to receptor binding by most RDR interference group members.<ref>{{cite journal |
The largest ectodomain of ASCT1 and ASCT2, extracellular loop 2 (ECL2), contains at its C-terminus a 21 residue hypervariable region between human, mouse, and hamster receptors. This region was shown to confer specificity to receptor binding by most RDR interference group members.<ref>{{cite journal|date=March 2003|title=N-linked glycosylation and sequence changes in a critical negative control region of the ASCT1 and ASCT2 neutral amino acid transporters determine their retroviral receptor functions|journal=Journal of Virology|volume=77|issue=5|pages=2936–45|doi=10.1128/JVI.77.5.2936-2945.2003|pmc=149750|pmid=12584318|vauthors=Marin M, Lavillette D, Kelly SM, Kabat D}}</ref> Both glycosylation pattern and amino acid sequence differences between human and rodent receptors are determinants in susceptibility to infection by RDR interference group members. Murine (mouse) ASCT1 expressing cells are only susceptible to syncytin-1 and another endogenous retroviral env protein (that of Baboon Endogenous Retrovrius) and human ASCT1 has only been shown to bind syncytin-1.<ref>{{cite journal|date=March 2003|title=N-linked glycosylation and sequence changes in a critical negative control region of the ASCT1 and ASCT2 neutral amino acid transporters determine their retroviral receptor functions|journal=Journal of Virology|volume=77|issue=5|pages=2936–45|doi=10.1128/JVI.77.5.2936-2945.2003|pmc=149750|pmid=12584318|vauthors=Marin M, Lavillette D, Kelly SM, Kabat D}}</ref> Further research is needed to elucidate ASCT and RDR binding determinants. |
||
== Structure == |
== Structure == |
||
Syncytin-1 shares many structural elements with class I retroviral glycoproteins (such as, [[Murine leukemia virus|Murine Leukemia Virus]] gp, [[Ebola virus|Ebolavirus]] gp, and [[HIV]] [[Envelope glycoprotein GP120|gp120]], [[gp41]]). It is composed of a surface subunit (SU) and transmembrane subunit (TM), separated by a [[furin]] cleavage site.<ref>{{cite journal |
Syncytin-1 shares many structural elements with class I retroviral glycoproteins (such as, [[Murine leukemia virus|Murine Leukemia Virus]] gp, [[Ebola virus|Ebolavirus]] gp, and [[HIV]] [[Envelope glycoprotein GP120|gp120]], [[gp41]]). It is composed of a surface subunit (SU) and transmembrane subunit (TM), separated by a [[furin]] cleavage site.<ref>{{cite journal|date=May 2005|title=Synthesis, assembly, and processing of the Env ERVWE1/syncytin human endogenous retroviral envelope|journal=Journal of Virology|volume=79|issue=9|pages=5585–93|doi=10.1128/JVI.79.9.5585-5593.2005|pmc=1082723|pmid=15827173|vauthors=Cheynet V, Ruggieri A, Oriol G, Blond JL, Boson B, Vachot L, Verrier B, Cosset FL, Mallet F}}</ref> The two subunits form a heterodimer and are likely linked by a disulfide bond between two conserved cysteine rich motifs: CXXC in SU and CX<sub>6</sub>CC in TM.<ref>{{cite journal|date=May 2005|title=Synthesis, assembly, and processing of the Env ERVWE1/syncytin human endogenous retroviral envelope|journal=Journal of Virology|volume=79|issue=9|pages=5585–93|doi=10.1128/JVI.79.9.5585-5593.2005|pmc=1082723|pmid=15827173|vauthors=Cheynet V, Ruggieri A, Oriol G, Blond JL, Boson B, Vachot L, Verrier B, Cosset FL, Mallet F}}</ref> This heterodimer likely forms a homotrimer at the cell surface. Syncytin-1 TM contains the fusion peptide, and two [[heptad repeat]]s separated by a chain reversal region common to class I retroviral glycoproteins. Syncytin-1 is a single pass [[membrane protein]] and has a relatively long [[cytoplasm]]ic tail; however, truncation of the cytoplasmic tail to just 14 [[Amino acid|residues]] has been shown to increase fusogenic activity, indicating its C-terminus is likely involved in modulating fusion activity.<ref>{{cite journal|date=August 2006|title=C-Terminal truncations of syncytin-1 (ERVWE1 envelope) that increase its fusogenicity|journal=Biological Chemistry|volume=387|issue=8|pages=1113–20|doi=10.1515/BC.2006.137|pmid=16895482|vauthors=Drewlo S, Leyting S, Kokozidou M, Mallet F, Pötgens AJ}}</ref> |
||
| ⚫ | |||
| ⚫ | Synyctin-1 mediated [[trophoblast]] fusion is essential for normal [[placenta]]l development. The placenta is composed on two cell layers: [[cytotrophoblast]] and [[syncytiotrophoblast]] layer. Cytotrophoblasts are continually dividing, non-differentiated cells and syncytiotrophoblasts are fully differentiated, non dividing, fused cells Syncytin-1 expression on the surface of cytotrophoblasts and syncytiotrophoblasts mediate fusion. The syncytiotrophoblast layer is the necessary interface between the developing embryo and the maternal blood supply, allowing nutrient and waste exchange and blocking maternal immune cell invasion, preventing immune rejection of the foetus. Syncytiotrophoblasts are forced into [[senescence]] by fusion.<ref>{{cite journal | vauthors = Chuprin A, Gal H, Biron-Shental T, Biran A, Amiel A, Rozenblatt S, Krizhanovsky V | title = Cell fusion induced by ERVWE1 or measles virus causes cellular senescence | journal = Genes & Development | volume = 27 | issue = 21 | pages = 2356–66 | date = November 2013 | pmid = 24186980 | pmc = 3828521 | doi = 10.1101/gad.227512.113 }}</ref> Therefore, cytotrophoblast proliferation is necessary for growth and maintenance of the syncytiotrophoblast layer. Syncytin-1 expression in cytotrophoblasts promotes G1/S transition and proliferation thereby ensuring continual replenishment of the cytotrophoblast pool.<ref>{{cite journal | vauthors = Huang Q, Li J, Wang F, Oliver MT, Tipton T, Gao Y, Jiang SW | title = Syncytin-1 modulates placental trophoblast cell proliferation by promoting G1/S transition | journal = Cellular Signalling | volume = 25 | issue = 4 | pages = 1027–35 | date = April 2013 | pmid = 23333240 | pmc = 4644426 | doi = 10.1016/j.cellsig.2013.01.008 }}</ref> The name syncytin derives from its involvement in the formation of syncytium, the multinucleated syncytiotrophoblast protoplasm. There is another endogenous retroviral envelope protein expressed in the placenta from a different ERV family: syncytin-2 (of [[HERV-FRD]]). |
||
== Clinical significance == |
== Clinical significance == |
||
Revision as of 21:52, 30 November 2016
| ERVW-1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | ERVW-1, ENV, ENVW, ERVWE1, HERV-7q, HERV-W-ENV, HERV7Q, HERVW, HERVWENV, endogenous retrovirus group W member 1, endogenous retrovirus group W member 1, envelope | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 604659; HomoloGene: 137309; GeneCards: ERVW-1; OMA:ERVW-1 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Syncytin-1 also known as enverin is a human fusion protein encoded by a human endogenous retroviral (HERV) gene: ERVWe-1. As an endogenous viral element, ERVWe1 is the remnant of an ancient retroviral infection integrated into the human germ line, analogous to the incorporation of certain bacterial species into animal cells that over the course of evolution eventually developed into mitochondria. In the case of syncytin-1, this integration likely occurred more than 25 million years ago.[3] ERVWe1 is the only known protein encoding member of the HERV-W family.
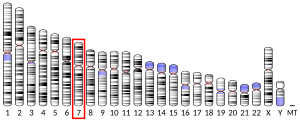
ERVWe1 is located within a full length provirus on chromosome 7 at locus 7q21.2 flanked by long terminal repeats (LTRs) and is preceded by ERVW1 gag (Group AntiGen) and pol (POLmerase) within the provirus, both of which contain nonsense mutations rendering them non-coding[4][5].
Syncytin-1 aids in embryo attachment to the uterus and establishment of a nutrient supply through the placenta. Gene knockout of syncytin genes in mice provides evidence for their absolute requirement for placenta development and embryo survival.[6] Syncytin-1 is also implicated in a number of neurological pathologies, most notably, multiple sclerosis, as an immunogen.
Syncytin-1 is one of two known syncytin proteins expressed in humans and one of many syncytins captured and domesticated on multiple occasions over evolutionary time in diverse mammalian species[7].
Receptor
The syncytin-1 receptor is the Na-dependent amino acid transporter 2 (ASCT2 or SLC1A5).[8][9] This receptor places syncytin-1 in a large viral interference group called retroviral mammalian type D receptor (RDR) interference group.[10] Syncytin-1 has been shown to interfere with viral infection in-vitro by RDR interference group member spleen necrosis virus.[11] Syncytin-1 can also recognize ASCT1 or SLC1A4, but this receptor is not a receptor for the RDR interference group. Mutation studies of syncytin-1 and of ASCT2 have provided insight into potential receptor binding domains and determinants. A putative receptor binding domain was identified in syncytin-1 at residues 117-144.[12] The amino acid sequence at this region is well conserved amongst RDR interference group members. The motif SDGGGX2DX2R is present in all RDR interference group members within this conserved region and may play an important role in binding. Preliminary evidence with syncytin-1 and spleen necrosis virus indicate this motif contains the ASCT2 binding determinants.[13][14][15]
The largest ectodomain of ASCT1 and ASCT2, extracellular loop 2 (ECL2), contains at its C-terminus a 21 residue hypervariable region between human, mouse, and hamster receptors. This region was shown to confer specificity to receptor binding by most RDR interference group members.[16] Both glycosylation pattern and amino acid sequence differences between human and rodent receptors are determinants in susceptibility to infection by RDR interference group members. Murine (mouse) ASCT1 expressing cells are only susceptible to syncytin-1 and another endogenous retroviral env protein (that of Baboon Endogenous Retrovrius) and human ASCT1 has only been shown to bind syncytin-1.[17] Further research is needed to elucidate ASCT and RDR binding determinants.
Structure
Syncytin-1 shares many structural elements with class I retroviral glycoproteins (such as, Murine Leukemia Virus gp, Ebolavirus gp, and HIV gp120, gp41). It is composed of a surface subunit (SU) and transmembrane subunit (TM), separated by a furin cleavage site.[18] The two subunits form a heterodimer and are likely linked by a disulfide bond between two conserved cysteine rich motifs: CXXC in SU and CX6CC in TM.[19] This heterodimer likely forms a homotrimer at the cell surface. Syncytin-1 TM contains the fusion peptide, and two heptad repeats separated by a chain reversal region common to class I retroviral glycoproteins. Syncytin-1 is a single pass membrane protein and has a relatively long cytoplasmic tail; however, truncation of the cytoplasmic tail to just 14 residues has been shown to increase fusogenic activity, indicating its C-terminus is likely involved in modulating fusion activity.[20]
Placental development
Synyctin-1 mediated trophoblast fusion is essential for normal placental development. The placenta is composed on two cell layers: cytotrophoblast and syncytiotrophoblast layer. Cytotrophoblasts are continually dividing, non-differentiated cells and syncytiotrophoblasts are fully differentiated, non dividing, fused cells Syncytin-1 expression on the surface of cytotrophoblasts and syncytiotrophoblasts mediate fusion. The syncytiotrophoblast layer is the necessary interface between the developing embryo and the maternal blood supply, allowing nutrient and waste exchange and blocking maternal immune cell invasion, preventing immune rejection of the foetus. Syncytiotrophoblasts are forced into senescence by fusion.[21] Therefore, cytotrophoblast proliferation is necessary for growth and maintenance of the syncytiotrophoblast layer. Syncytin-1 expression in cytotrophoblasts promotes G1/S transition and proliferation thereby ensuring continual replenishment of the cytotrophoblast pool.[22] The name syncytin derives from its involvement in the formation of syncytium, the multinucleated syncytiotrophoblast protoplasm. There is another endogenous retroviral envelope protein expressed in the placenta from a different ERV family: syncytin-2 (of HERV-FRD).
Clinical significance
Pre-eclampsia
Hypoxic conditions characteristic of Pre-eclampsia and IUGR are associated with abnormal expression of syncytin-1 in trophoblast cells[23] and pre-eclamptic placental tissue has reduced levels of syncytin-1 expression.[24] Abnormal syncytin-1 expression likely plays an important role in placental pathologies.
Neurological pathologies
ERVWe1 is a single locus within the HERV-W family encoding a fully functional env protein. mRNA and protein expression of the ERVWe1 locus in neural tissue is implicated in neurodegeneration and development of multiple sclerosis. Multiple sclerosis retrovirus like particle (MSRV) envelope protein shares high sequence similarity to ERVWe1 encoded syncytin-1 and has long been studied as an important factor in MS pathogenesis.[25] The gene locus of MSRV env has not been determined.
Preliminary evidence implicates aberrant expression of ERVWe1 in neuron and glial cells and HERV-W LTR mediated aberrant cellular protein expression in the pathogenesis of bipolar disorder and schizophrenia[26][27]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000242950 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Voisset C, Blancher A, Perron H, Mandrand B, Mallet F, Paranhos-Baccalà G (November 1999). "Phylogeny of a novel family of human endogenous retrovirus sequences, HERV-W, in humans and other primates". AIDS Research and Human Retroviruses. 15 (17): 1529–33. doi:10.1089/088922299309810. PMID 10580403.
- ^ "ERVW-1 endogenous retrovirus group W member 1 [Homo sapiens (human)]". Gene - NCBI. Retrieved 2016-11-23.
- ^ Voisset C, Bouton O, Bedin F, Duret L, Mandrand B, Mallet F, Paranhos-Baccala G (May 2000). "Chromosomal distribution and coding capacity of the human endogenous retrovirus HERV-W family". AIDS Research and Human Retroviruses. 16 (8): 731–40. doi:10.1089/088922200308738. PMID 10826480.
- ^ Dupressoir A, Lavialle C, Heidmann T (2012). "From ancestral infectious retroviruses to bona fide cellular genes: role of the captured syncytins in placentation". Placenta. 33 (9): 663–71. doi:10.1016/j.placenta.2012.05.005. PMID 22695103.
- ^ Lavialle C, Cornelis G, Dupressoir A, Esnault C, Heidmann O, Vernochet C, Heidmann T (2013). "Paleovirology of 'syncytins', retroviral env genes exapted for a role in placentation". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 368 (1626): 20120507. doi:10.1098/rstb.2012.0507. PMC 3758191. PMID 23938756.
- ^ Lavillette D, Marin M, Ruggieri A, Mallet F, Cosset FL, Kabat D (July 2002). "The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors". Journal of Virology. 76 (13): 6442–52. doi:10.1128/JVI.76.13.6442-6452.2002. PMC 136247. PMID 12050356.
- ^ Blond JL, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel-Fernandes S, Mandrand B, Mallet F, Cosset FL (April 2000). "An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor". Journal of Virology. 74 (7): 3321–9. PMC 111833. PMID 10708449.
- ^ Sommerfelt MA, Weiss RA (May 1990). "Receptor interference groups of 20 retroviruses plating on human cells". Virology. 176 (1): 58–69. doi:10.1016/0042-6822(90)90230-O. PMID 1691887.
- ^ Ponferrada VG, Mauck BS, Wooley DP (April 2003). "The envelope glycoprotein of human endogenous retrovirus HERV-W induces cellular resistance to spleen necrosis virus". Archives of Virology. 148 (4): 659–75. doi:10.1007/s00705-002-0960-x. PMID 12664292.
- ^ Slokar G, Hasler G (2015-01-01). "Human Endogenous Retroviruses as Pathogenic Factors in the Development of Schizophrenia". Frontiers in Psychiatry. 6: 183. doi:10.3389/fpsyt.2015.00183. PMC 4707225. PMID 26793126.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Slokar G, Hasler G (2015-01-01). "Human Endogenous Retroviruses as Pathogenic Factors in the Development of Schizophrenia". Frontiers in Psychiatry. 6: 183. doi:10.3389/fpsyt.2015.00183. PMC 4707225. PMID 26793126.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Martinez I, Dornburg R (July 1995). "Mapping of receptor binding domains in the envelope protein of spleen necrosis virus". Journal of Virology. 69 (7): 4339–46. PMC 189174. PMID 7769695.
- ^ Martinez I, Dornburg R (September 1996). "Mutational analysis of the envelope protein of spleen necrosis virus". Journal of Virology. 70 (9): 6036–43. PMC 190624. PMID 8709226.
- ^ Marin M, Lavillette D, Kelly SM, Kabat D (March 2003). "N-linked glycosylation and sequence changes in a critical negative control region of the ASCT1 and ASCT2 neutral amino acid transporters determine their retroviral receptor functions". Journal of Virology. 77 (5): 2936–45. doi:10.1128/JVI.77.5.2936-2945.2003. PMC 149750. PMID 12584318.
- ^ Marin M, Lavillette D, Kelly SM, Kabat D (March 2003). "N-linked glycosylation and sequence changes in a critical negative control region of the ASCT1 and ASCT2 neutral amino acid transporters determine their retroviral receptor functions". Journal of Virology. 77 (5): 2936–45. doi:10.1128/JVI.77.5.2936-2945.2003. PMC 149750. PMID 12584318.
- ^ Cheynet V, Ruggieri A, Oriol G, Blond JL, Boson B, Vachot L, Verrier B, Cosset FL, Mallet F (May 2005). "Synthesis, assembly, and processing of the Env ERVWE1/syncytin human endogenous retroviral envelope". Journal of Virology. 79 (9): 5585–93. doi:10.1128/JVI.79.9.5585-5593.2005. PMC 1082723. PMID 15827173.
- ^ Cheynet V, Ruggieri A, Oriol G, Blond JL, Boson B, Vachot L, Verrier B, Cosset FL, Mallet F (May 2005). "Synthesis, assembly, and processing of the Env ERVWE1/syncytin human endogenous retroviral envelope". Journal of Virology. 79 (9): 5585–93. doi:10.1128/JVI.79.9.5585-5593.2005. PMC 1082723. PMID 15827173.
- ^ Drewlo S, Leyting S, Kokozidou M, Mallet F, Pötgens AJ (August 2006). "C-Terminal truncations of syncytin-1 (ERVWE1 envelope) that increase its fusogenicity". Biological Chemistry. 387 (8): 1113–20. doi:10.1515/BC.2006.137. PMID 16895482.
- ^ Chuprin A, Gal H, Biron-Shental T, Biran A, Amiel A, Rozenblatt S, Krizhanovsky V (November 2013). "Cell fusion induced by ERVWE1 or measles virus causes cellular senescence". Genes & Development. 27 (21): 2356–66. doi:10.1101/gad.227512.113. PMC 3828521. PMID 24186980.
- ^ Huang Q, Li J, Wang F, Oliver MT, Tipton T, Gao Y, Jiang SW (April 2013). "Syncytin-1 modulates placental trophoblast cell proliferation by promoting G1/S transition". Cellular Signalling. 25 (4): 1027–35. doi:10.1016/j.cellsig.2013.01.008. PMC 4644426. PMID 23333240.
- ^ Wich C, Kausler S, Dotsch J, Rascher W, Knerr I (2009-01-01). "Syncytin-1 and glial cells missing a: hypoxia-induced deregulated gene expression along with disordered cell fusion in primary term human trophoblasts". Gynecologic and Obstetric Investigation. 68 (1): 9–18. doi:10.1159/000209396. PMID 19321927.
- ^ Holder BS, Tower CL, Abrahams VM, Aplin JD (June 2012). "Syncytin 1 in the human placenta". Placenta. 33 (6): 460–6. doi:10.1016/j.placenta.2012.02.012. PMID 22381536.
- ^ Laufer G, Mayer J, Mueller BF, Mueller-Lantzsch N, Ruprecht K (April 2009). "Analysis of transcribed human endogenous retrovirus W env loci clarifies the origin of multiple sclerosis-associated retrovirus env sequences". Retrovirology. 6: 37. doi:10.1186/1742-4690-6-37. PMC 2672075. PMID 19368703.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Slokar G, Hasler G (2015-01-01). "Human Endogenous Retroviruses as Pathogenic Factors in the Development of Schizophrenia". Frontiers in Psychiatry. 6: 183. doi:10.3389/fpsyt.2015.00183. PMC 4707225. PMID 26793126.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Karlsson H, Schröder J, Bachmann S, Bottmer C, Yolken RH (January 2004). "HERV-W-related RNA detected in plasma from individuals with recent-onset schizophrenia or schizoaffective disorder". Molecular Psychiatry. 9 (1): 12–3. doi:10.1038/sj.mp.4001439. PMID 14571258.
{{cite journal}}: Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help)