Addiction: Difference between revisions
c |
→Age: Deleted sentence that was direct quote (as is previous sentence) of unsubstantiated web page that it cites. On their face the stats in the previous sentence do not without knowing where the remaining % lie, do what the deleted sentence claims. |
||
| Line 44: | Line 44: | ||
=== Age === |
=== Age === |
||
Adolescence represents a period of unique vulnerability for developing addiction.<ref>{{cite journal | vauthors = Spear LP | title = The adolescent brain and age-related behavioral manifestations | journal = Neuroscience and Biobehavioral Reviews | volume = 24 | issue = 4 | pages = 417–463 | date = June 2000 | pmid = 10817843 | doi = 10.1016/s0149-7634(00)00014-2 }}</ref> Not only are adolescents more likely to initiate and maintain drug use, but once addicted they are more resistant to treatment and more liable to relapse.<ref>{{cite journal | vauthors = Catalano RF, Hawkins JD, Wells EA, Miller J, Brewer D | title = Evaluation of the effectiveness of adolescent drug abuse treatment, assessment of risks for relapse, and promising approaches for relapse prevention | journal = The International Journal of the Addictions | volume = 25 | issue = 9A-10A | pages = 1085–140 | pmid = 2131328 | year=1990}}</ref><ref>{{cite journal | vauthors = Perepletchikova F, Krystal JH, Kaufman J | title = Practitioner review: adolescent alcohol use disorders: assessment and treatment issues | journal = Journal of Child Psychology and Psychiatry, and Allied Disciplines | volume = 49 | issue = 11 | pages = 1131–1154 | date = November 2008 | pmid = 19017028 | pmc = 4113213 | doi = 10.1111/j.1469-7610.2008.01934.x }}</ref> Statistics have shown that those who start to drink alcohol at a younger age are more likely to become dependent later on. About 33% of the population tasted their first alcohol between the ages of 15 and 17, while 18% experienced it prior to this. As for alcohol abuse or dependence, the numbers start off high with those who first drank before they were 12 and then drop off after that. For example, 16% of alcoholics began drinking prior to turning 12 years old, while only 9% first touched alcohol between 15 and 17. This percentage is even lower, at 2.6%, for those who first started the habit after they were 21 |
Adolescence represents a period of unique vulnerability for developing addiction.<ref>{{cite journal | vauthors = Spear LP | title = The adolescent brain and age-related behavioral manifestations | journal = Neuroscience and Biobehavioral Reviews | volume = 24 | issue = 4 | pages = 417–463 | date = June 2000 | pmid = 10817843 | doi = 10.1016/s0149-7634(00)00014-2 }}</ref> Not only are adolescents more likely to initiate and maintain drug use, but once addicted they are more resistant to treatment and more liable to relapse.<ref>{{cite journal | vauthors = Catalano RF, Hawkins JD, Wells EA, Miller J, Brewer D | title = Evaluation of the effectiveness of adolescent drug abuse treatment, assessment of risks for relapse, and promising approaches for relapse prevention | journal = The International Journal of the Addictions | volume = 25 | issue = 9A-10A | pages = 1085–140 | pmid = 2131328 | year=1990}}</ref><ref>{{cite journal | vauthors = Perepletchikova F, Krystal JH, Kaufman J | title = Practitioner review: adolescent alcohol use disorders: assessment and treatment issues | journal = Journal of Child Psychology and Psychiatry, and Allied Disciplines | volume = 49 | issue = 11 | pages = 1131–1154 | date = November 2008 | pmid = 19017028 | pmc = 4113213 | doi = 10.1111/j.1469-7610.2008.01934.x }}</ref> Statistics have shown that those who start to drink alcohol at a younger age are more likely to become dependent later on. About 33% of the population tasted their first alcohol between the ages of 15 and 17, while 18% experienced it prior to this. As for alcohol abuse or dependence, the numbers start off high with those who first drank before they were 12 and then drop off after that. For example, 16% of alcoholics began drinking prior to turning 12 years old, while only 9% first touched alcohol between 15 and 17. This percentage is even lower, at 2.6%, for those who first started the habit after they were 21.<ref>http://alcoholrehab.com/drug-addiction/age-and-substance-abuse/</ref> |
||
=== Genetic factors === |
=== Genetic factors === |
||
Revision as of 05:12, 1 February 2017
| ||||||
| ||||||
Addiction is a brain disorder characterized by compulsive engagement in rewarding stimuli, despite adverse consequences.[8] Despite the involvement of a number of psychosocial factors, a biological process – one which is induced by repeated exposure to an addictive stimulus – is the core pathology that drives the development and maintenance of an addiction.[1][9] The two properties that characterize all addictive stimuli are that they are reinforcing (i.e., they increase the likelihood that a person will seek repeated exposure to them) and intrinsically rewarding (i.e., perceived as being positive or desirable).[1][4][7]
Addiction is a disorder of the brain's reward system which arises through transcriptional and epigenetic mechanisms and occurs over time from chronically high levels of exposure to an addictive stimulus (e.g., morphine, cocaine, sexual intercourse, gambling, etc.).[1][10][11] ΔFosB, a gene transcription factor, is a critical component and common factor in the development of virtually all forms of behavioral and drug addictions.[10][11][12][13] Two decades of research into ΔFosB's role in addiction have demonstrated that addiction arises, and the associated compulsive behavior intensifies or attenuates, along with the genetic overexpression of ΔFosB in the D1-type medium spiny neurons of the nucleus accumbens.[1][10][11][12] Due to the causal relationship between ΔFosB expression and addictions, it is used preclinically as an addiction biomarker.[1][10][12] ΔFosB expression in these neurons directly and positively regulates drug self-administration and reward sensitization through positive reinforcement, while decreasing sensitivity to aversion.[note 1][1][10]
Addiction exacts a high toll on individuals and society as a whole through the direct adverse effects of drugs, associated healthcare costs, long-term complications (e.g., lung cancer with smoking tobacco, liver cirrhosis with drinking alcohol, or meth mouth from intravenous methamphetamine), the functional consequences of altered neural plasticity in the brain, and the consequent loss of productivity.[14][15][16] Classic hallmarks of addiction include impaired control over substances or behavior, preoccupation with substance or behavior, and continued use despite consequences.[17] Habits and patterns associated with addiction are typically characterized by immediate gratification (short-term reward), coupled with delayed deleterious effects (long-term costs).[18]
Examples of drug and behavioral addictions include: alcoholism, amphetamine addiction, cocaine addiction, nicotine addiction, opiate addiction, food addiction, gambling addiction, and sexual addiction. The only behavioral addiction recognized by the DSM-5 is gambling addiction. The term addiction is misused frequently to refer to other compulsive behaviors or disorders, particularly dependence, in news media.[19] An important distinction between drug addiction and dependence is that drug dependence is a disorder in which cessation of drug use results in an unpleasant state of withdrawal, which can lead to further drug use. Addiction is the compulsive use of a substance or performance of a behavior that is independent of withdrawal.
Neuropsychology
This section needs expansion. You can help by adding to it. (February 2016) |
Cognitive control and stimulus control, which is associated with operant and classical conditioning, represent opposite processes (i.e., internal vs external or environmental, respectively) that compete over the control of an individual's elicited behaviors.[20] Cognitive control, and particularly inhibitory control over behavior, is impaired in both addiction and attention deficit hyperactivity disorder.[21][22] Stimulus-driven behavioral responses (i.e., stimulus control) that are associated with a particular rewarding stimulus tend to dominate one's behavior in an addiction.[22]
Stimulus control of behavior
Cognitive control of behavior
Behavioral addiction
The term behavioral addiction correctly refers to a compulsion to engage in a natural reward – which is a behavior that is inherently rewarding (i.e., desirable or appealing) – despite adverse consequences.[6][11][13] Preclinical evidence has demonstrated that overexpression of ΔFosB through repetitive and excessive exposure to a natural reward induces the same behavioral effects and neuroplasticity as occurs in a drug addiction.[11][23][24][25]
Reviews of both clinical research in humans and preclinical studies involving ΔFosB have identified compulsive sexual activity – specifically, any form of sexual intercourse – as an addiction (i.e., sexual addiction); moreover, reward cross-sensitization between amphetamine and sexual activity, a property in which exposure to one increases in the desire for both, has been shown to occur preclinically and clinically as a dopamine dysregulation syndrome;[11][23][24][25] ΔFosB expression is required for this cross-sensitization effect, which intensifies with the level of ΔFosB expression.[11][24][25]
Reviews of preclinical studies indicate that long-term frequent and excessive consumption of high fat or sugar foods can produce an addiction (food addiction).[11][13]
Gambling is a natural reward which is associated with compulsive behavior and for which clinical diagnostic manuals, namely the DSM-5, have identified diagnostic criteria for an "addiction".[11] There is evidence from functional neuroimaging that gambling activates the reward system and the mesolimbic pathway in particular.[11][26] Similarly, shopping and playing videogames are associated with compulsive behaviors in humans and have also been shown to activate the mesolimbic pathway and other parts of the reward system.[11] Based upon this evidence, gambling addiction, video game addiction and shopping addiction are classified accordingly.[11][26]
Risk factors
There are a range of genetic and environmental risk factors for developing an addiction that vary across the population.[1][27] Roughly half of an individual's risk for developing an addiction is derived from genetics, while the other half is derived from the environment.[1] However, even in individuals with a relatively low genetic loading, exposure to sufficiently high doses of an addictive drug for a long period of time (e.g., weeks–months) can result in an addiction.[1] In other words, anyone can become an addict under the right circumstances.
Age
Adolescence represents a period of unique vulnerability for developing addiction.[28] Not only are adolescents more likely to initiate and maintain drug use, but once addicted they are more resistant to treatment and more liable to relapse.[29][30] Statistics have shown that those who start to drink alcohol at a younger age are more likely to become dependent later on. About 33% of the population tasted their first alcohol between the ages of 15 and 17, while 18% experienced it prior to this. As for alcohol abuse or dependence, the numbers start off high with those who first drank before they were 12 and then drop off after that. For example, 16% of alcoholics began drinking prior to turning 12 years old, while only 9% first touched alcohol between 15 and 17. This percentage is even lower, at 2.6%, for those who first started the habit after they were 21.[31]
Genetic factors
It has long been established that genetic factors along with social and psychological factors are contributors to addiction. A common theory along these lines is the self-medication hypothesis. Epidemiological studies estimate that genetic factors account for 40–60% of the risk factors for alcoholism. Similar rates of heritability for other types of drug addiction have been indicated by other studies.[32] Knestler hypothesized in 1964 that a gene or group of genes might contribute to predisposition to addiction in several ways. For example, altered levels of a normal protein due to environmental factors could then change the structure or functioning of specific brain neurons during development. These altered brain neurons could change the susceptibility of an individual to an initial drug use experience. In support of this hypothesis, animal studies have shown that environmental factors such as stress can affect an animal's genotype.[32]
Overall, the data implicating specific genes in the development of drug addiction is mixed for most genes. One reason for this may be that the case is due to a focus of current research on common variants. Many addiction studies focus on common variants with an allele frequency of greater than 5% in the general population, however when associated with disease, these only confer a small amount of additional risk with an odds ratio of 1.1–1.3 percent. On the other hand, the rare variant hypothesis states that genes with low frequencies in the population (<1%) confer much greater additional risk in the development of disease.[33]
Genome-wide association studies (GWAS) are a recently developed research method which are used to examine genetic associations with dependence, addiction, and drug use. These studies employ an unbiased approach to finding genetic associations with specific phenotypes and give equal weight to all regions of DNA, including those with no ostensible relationship to drug metabolism or response. These studies rarely identify genes from proteins previously described via animal knockout models and candidate gene analysis. Instead, large percentages of genes involved in processes such as cell adhesion are commonly identified. This is not to say that previous findings, or the GWAS findings, are erroneous. The important effects of endophenotypes are typically not capable of being captured by these methods. Furthermore, genes identified in GWAS for drug addiction may be involved either in adjusting brain behavior prior to drug experiences, subsequent to them, or both. [34]
Environmental factors
Adverse childhood experiences (ACEs) are various forms of maltreatment and household dysfunction experienced in childhood. The Adverse Childhood Experiences Study by the Centers for Disease Control and Prevention has shown a strong dose–response relationship between ACEs and numerous health, social, and behavioral problems throughout a person's lifespan, including those associated with substance abuse.[35] Children's neurological development can be disrupted when they are chronically exposed to stressful events such as physical, emotional, or sexual abuse, physical or emotional neglect, witnessing violence in the household, or a parent being incarcerated or suffering from a mental illness. As a result, the child's cognitive functioning or ability to cope with negative or disruptive emotions may be impaired. Over time, the child may adopt substance use as a coping mechanism, particularly during adolescence.[35]
The National Institute on Drug Abuse cites lack of parental supervision, the prevalence of peer substance use, drug availability, and poverty as risk factors for substance use development.[36]
Psychological factors
Individuals with comorbid (i.e., co-occurring) mental health disorders such as depression, anxiety, attention-deficit/hyperactivity disorder (ADHD) or post-traumatic stress disorder are more likely to develop substance use disorders.[37][38][39]
The National Institute on Drug Abuse cites early aggressive behavior as a risk factor for substance use development.[40]
Transgenerational epigenetic inheritance
Epigenetic genes and their products (e.g., proteins) are the key components through which environmental influences can affect the genes of an individual;[27] they also serve as the mechanism responsible for the transgenerational epigenetic inheritance of behavioral phenotypes, a phenomenon in which environmental influences on the genes of a parent can affect the associated traits and behavioral phenotypes of their offspring (e.g., behavioral responses to certain environmental stimuli).[27] In addiction, epigenetic mechanisms play a central role in the pathophysiology of the disease;[1] it has been noted that some of the alterations to the epigenome which arise through chronic exposure to addictive stimuli during an addiction can be transmitted across generations, in turn affecting the behavior of one's children (e.g., the child's behavioral responses to addictive drugs and natural rewards).[27][41] More research is needed to determine the specific epigenetic mechanisms and the nature of heritable behavioral phenotypes that arise from addictions in humans.[27][41] Based upon preclinical evidence with lab animals, the addiction-related behavioral phenotypes that are transmitted across generations may serve to increase or decrease the child's risk of developing an addiction.[27][41]
Mechanisms
| Transcription factor glossary | |
|---|---|
| |
Current models of addiction from chronic addictive drug use involve alterations in gene expression in the mesocorticolimbic projection.[13][49][50] The most important transcription factors that produce these alterations are ΔFosB, cAMP response element binding protein (CREB), and nuclear factor kappa B (NF-κB).[13] ΔFosB is the most significant gene transcription factor in addiction since its viral or genetic overexpression in the nucleus accumbens is necessary and sufficient for most of the behaviors and neural adaptations seen in drug addiction.[13] ΔFosB expression in nucleus accumbens D1-type medium spiny neurons directly and positively regulates drug self-administration and reward sensitization through positive reinforcement while decreasing sensitivity to aversion.[note 1][1][10] Specific drug addictions in which ΔFosB has been implicated in addictions to alcohol, amphetamine, cannabinoids, cocaine, methylphenidate, nicotine, phenylcyclidine, propofol, opiates, and substituted amphetamines, among others.[10][13][49][51][52] ΔJunD (a transcription factor) and G9a (an epigenetic enzyme) directly oppose ΔFosB's expression and function.[12][13] Increases in nucleus accumbens ΔJunD or G9a expression using viral vectors can reduce or, with a large increase, even block and reverse many of the neural and behavioral alterations seen in chronic drug abuse (i.e., the alterations mediated by ΔFosB).[12][13]
ΔFosB also plays an important role in regulating behavioral responses to natural rewards, such as palatable food, sex, and exercise.[13][53] Natural rewards, like drugs of abuse, induce gene expression of ΔFosB in the nucleus accumbens, and chronic acquisition of these rewards can result in a similar pathological addictive state through ΔFosB overexpression.[11][13][53] Consequently, ΔFosB is the key transcription factor involved in addictions to natural rewards (i.e., behavioral addictions) as well;[13][11][53] in particular, ΔFosB in the nucleus accumbens is critical for the reinforcing effects of sexual reward.[53] Research on the interaction between natural and drug rewards suggests that dopaminergic psychostimulants (e.g., amphetamine) and sexual behavior act on similar biomolecular mechanisms to induce ΔFosB in the nucleus accumbens and possess bidirectional cross-sensitization effects that are mediated through ΔFosB.[11][24][25] This phenomenon is notable since, in humans, a dopamine dysregulation syndrome, characterized by drug-induced compulsive engagement in natural rewards (specifically, sexual activity, shopping, and gambling), has also been observed in some individuals taking dopaminergic medications.[11]
ΔFosB inhibitors (drugs or treatments that oppose its action) may be an effective treatment for addiction and addictive disorders.[54]
The release of dopamine in the nucleus accumbens plays a role in the reinforcing qualities of many forms of stimuli, including naturally reinforcing stimuli like palatable food and sex.[55][56] Altered dopamine neurotransmission is frequently observed following the development of an addictive state.[11] In humans and lab animals that have developed an addiction, alterations in dopamine or opioid neurotransmission in the nucleus accumbens and other parts of the striatum are evident.[11] Studies have found that use of certain drugs (e.g., cocaine) affect cholinergic neurons that innervate the reward system, in turn affecting dopamine signaling in this region.[57]
Summary of addiction-related plasticity
| Form of neuroplasticity or behavioral plasticity |
Type of reinforcer | Sources | |||||
|---|---|---|---|---|---|---|---|
| Opiates | Psychostimulants | High fat or sugar food | Sexual intercourse | Physical exercise (aerobic) |
Environmental enrichment | ||
| ΔFosB expression in nucleus accumbens D1-type MSNs |
↑ | ↑ | ↑ | ↑ | ↑ | ↑ | [11] |
| Behavioral plasticity | |||||||
| Escalation of intake | Yes | Yes | Yes | [11] | |||
| Psychostimulant cross-sensitization |
Yes | Not applicable | Yes | Yes | Attenuated | Attenuated | [11] |
| Psychostimulant self-administration |
↑ | ↑ | ↓ | ↓ | ↓ | [11] | |
| Psychostimulant conditioned place preference |
↑ | ↑ | ↓ | ↑ | ↓ | ↑ | [11] |
| Reinstatement of drug-seeking behavior | ↑ | ↑ | ↓ | ↓ | [11] | ||
| Neurochemical plasticity | |||||||
| CREB phosphorylation in the nucleus accumbens |
↓ | ↓ | ↓ | ↓ | ↓ | [11] | |
| Sensitized dopamine response in the nucleus accumbens |
No | Yes | No | Yes | [11] | ||
| Altered striatal dopamine signaling | ↓DRD2, ↑DRD3 | ↑DRD1, ↓DRD2, ↑DRD3 | ↑DRD1, ↓DRD2, ↑DRD3 | ↑DRD2 | ↑DRD2 | [11] | |
| Altered striatal opioid signaling | No change or ↑μ-opioid receptors |
↑μ-opioid receptors ↑κ-opioid receptors |
↑μ-opioid receptors | ↑μ-opioid receptors | No change | No change | [11] |
| Changes in striatal opioid peptides | ↑dynorphin No change: enkephalin |
↑dynorphin | ↓enkephalin | ↑dynorphin | ↑dynorphin | [11] | |
| Mesocorticolimbic synaptic plasticity | |||||||
| Number of dendrites in the nucleus accumbens | ↓ | ↑ | ↑ | [11] | |||
| Dendritic spine density in the nucleus accumbens |
↓ | ↑ | ↑ | [11] | |||
Reward system
This section needs expansion. You can help by adding to it. (August 2015) |
Mesocorticolimbic pathway
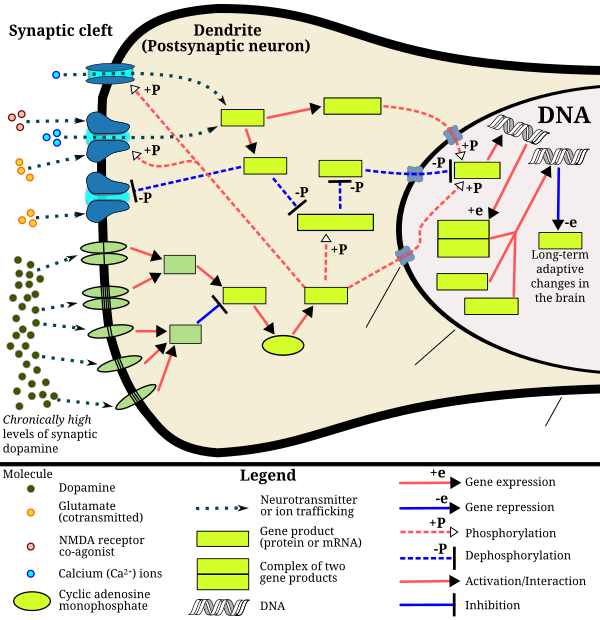
ΔFosB accumulation from excessive drug use
Top: this depicts the initial effects of high dose exposure to an addictive drug on gene expression in the nucleus accumbens for various genes in the Fos family.
Bottom: this illustrates the progressive increase in ΔFosB expression in the nucleus accumbens following repeated twice daily drug binges, where these phosphorylated (35–37 kD) ΔFosB isoforms persist in the D1-type medium spiny neurons of the nucleus accumbens for up to 2 months.[47][58] |
Understanding the pathways in which drugs act and how drugs can alter those pathways is key when examining the biological basis of drug addiction. The reward pathway, known as the mesolimbic pathway, or its extension, the mesocorticolimbic pathway, is characterized by the interaction of several areas of the brain.
- The projections from the ventral tegmental area (VTA) are a network of dopaminergic neurons with co-localized postsynaptic glutamate receptors (AMPAR and NMDAR). These cells respond when stimuli indicative of a reward are present. The VTA supports learning and sensitization development and releases DA into the forebrain.[59] These neurons also project and release DA into the nucleus accumbens,[60] through the mesolimbic pathway. Virtually all drugs causing drug addiction increase the dopamine release in the mesolimbic pathway,[61] in addition to their specific effects.
- The nucleus accumbens (NAcc) is one output of the VTA projections. The nucleus accumbens itself consists mainly of GABAergic medium spiny neurons (MSNs).[62] The NAcc is associated with acquiring and eliciting conditioned behaviors, and is involved in the increased sensitivity to drugs as addiction progresses.[59] Overexpression of ΔFosB in the nucleus accumbens is a necessary common factor in essentially all known forms of addiction;[1] ΔFosB is a strong positive modulator of positively reinforced behaviors.[1]
- The prefrontal cortex, including the anterior cingulate and orbitofrontal cortices,[63] is another VTA output in the mesocorticolimbic pathway; it is important for the integration of information which helps determine whether a behavior will be elicited.[64] It is also critical for forming associations between the rewarding experience of drug use and cues in the environment. Importantly, these cues are strong mediators of drug-seeking behavior and can trigger relapse even after months or years of abstinence.[65]
Other brain structures that are involved in addiction include:
- The basolateral amygdala projects into the NAcc and is thought to also be important for motivation.[64]
- The hippocampus is involved in drug addiction, because of its role in learning and memory. Much of this evidence stems from investigations showing that manipulating cells in the hippocampus alters dopamine levels in NAcc and firing rates of VTA dopaminergic cells.[60]
Role of dopamine and glutamate
Dopamine is the primary neurotransmitter of the reward system in the brain. It plays a role in regulating movement, emotion, cognition, motivation, and feelings of pleasure.[66] Natural rewards, like eating, as well as recreational drug use cause a release of dopamine, and are associated with the reinforcing nature of these stimuli.[66][67] Nearly all addictive drugs, directly or indirectly, act upon the brain's reward system by heightening dopaminergic activity.[68]
Excessive intake of many types of addictive drugs results in repeated release of high amounts of dopamine, which in turn affects the reward pathway directly through heightened dopamine receptor activation. Prolonged and abnormally high levels of dopamine in the synaptic cleft can induce receptor downregulation in the neural pathway. Downregulation of mesolimbic dopamine receptors can result in a decrease in the sensitivity to natural reinforcers.[66]
Drug seeking behavior is induced by glutamatergic projections from the prefrontal cortex to the nucleus accumbens. This idea is supported with data from experiments showing that drug seeking behavior can be prevented following the inhibition of AMPA glutamate receptors and glutamate release in the nucleus accumbens.[63]
Reward sensitization
| Target gene |
Target expression |
Neural effects | Behavioral effects |
|---|---|---|---|
| c-Fos | ↓ | Molecular switch enabling the chronic induction of ΔFosB[note 2] |
– |
| dynorphin | ↓ [note 3] |
• Downregulation of κ-opioid feedback loop | • Increased drug reward |
| NF-κB | ↑ | • Expansion of NAcc dendritic processes • NF-κB inflammatory response in the NAcc • NF-κB inflammatory response in the CP |
• Increased drug reward • Increased drug reward • Locomotor sensitization |
| GluR2 | ↑ | • Decreased sensitivity to glutamate | • Increased drug reward |
| Cdk5 | ↑ | • GluR1 synaptic protein phosphorylation • Expansion of NAcc dendritic processes |
Decreased drug reward (net effect) |
Reward sensitization is a process that causes an increase in the amount of reward (specifically, incentive salience[note 4]) that is assigned by the brain to a rewarding stimulus (e.g., a drug). In simple terms, when reward sensitization to a specific stimulus (e.g., a drug) occurs, an individual's "wanting" or desire for the stimulus itself and its associated cues increases.[71][70][72] Reward sensitization normally occurs following chronically high levels of exposure to the stimulus. ΔFosB (DeltaFosB) expression in D1-type medium spiny neurons in the nucleus accumbens has been shown to directly and positively regulate reward sensitization from drugs and natural rewards (i.e., higher levels of ΔFosB expression increases both drug reward and behavioral reward).[1][10][12]
"Cue-induced wanting" or "cue-triggered wanting", a form of craving that occurs in addiction, is responsible for the majority of compulsive behavior that addicts exhibit.[70][72] These cues create overwhelming short-term urges to engage an addictive stimulus by acting as secondary reinforcers for the addictive stimulus (a primary reinforcer) that are assigned pathologically high levels of incentive salience ("want").[70][72]
Research on the interaction between natural and drug rewards suggests that dopaminergic psychostimulants (e.g., amphetamine) and sexual behavior act on similar biomolecular mechanisms to induce ΔFosB in the nucleus accumbens and possess a bidirectional reward cross-sensitization effect[note 5] that is mediated through ΔFosB.[11][24][25]
In contrast to ΔFosB's reward-sensitizing effect, CREB transcriptional activity decreases user's sensitivity to the rewarding effects of the substance. CREB transcription in the nucleus accumbens is implicated in psychological dependence and symptoms involving a lack of pleasure or motivation during drug withdrawal.[1][58][69]
The set of proteins known as "regulators of G protein signaling" (RGS), particularly RGS4 and RGS9-2, have been implicated in modulating some forms of opioid sensitization, including reward sensitization.[73]
Diagnosis
The 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) uses the term "Substance Use Disorder" to refer to a spectrum of use-related conditions. The DSM-5 eliminates the terms "abuse" and "dependence" from diagnostic categories, instead using the specifiers of "mild", "moderate" and "severe" to indicate the extent of disordered use. Specifiers are determined by the number of diagnostic criteria present in a given case. The manual has never actually used the term "addiction" clinically.[19] Currently, only drug addictions and gambling addiction are listed in the DSM-5. Past editions have used physical dependence and the associated withdrawal syndrome to identify an addictive state. Physical dependence occurs when the body has adjusted by incorporating the substance into its "normal" functioning – i.e., attains homeostasis – and therefore physical withdrawal symptoms occur upon cessation of use.[74] Tolerance is the process by which the body continually adapts to the substance and requires increasingly larger amounts to achieve the original effects. Withdrawal refers to physical and psychological symptoms experienced when reducing or discontinuing a substance that the body has become dependent on. Symptoms of withdrawal generally include but are not limited to anxiety, irritability, intense cravings for the substance, nausea, hallucinations, headaches, cold sweats, and tremors.
Medical researchers who actively study addiction have criticized the DSM classification of addiction for being flawed and involving arbitrary diagnostic criteria.[75] Writing in 2013, the director of the United States National Institute of Mental Health discussed the invalidity of the DSM-5's classification of mental disorders:[76]
While DSM has been described as a "Bible" for the field, it is, at best, a dictionary, creating a set of labels and defining each. The strength of each of the editions of DSM has been "reliability" – each edition has ensured that clinicians use the same terms in the same ways. The weakness is its lack of validity. Unlike our definitions of ischemic heart disease, lymphoma, or AIDS, the DSM diagnoses are based on a consensus about clusters of clinical symptoms, not any objective laboratory measure. In the rest of medicine, this would be equivalent to creating diagnostic systems based on the nature of chest pain or the quality of fever.
Most recently, though, the NIH acknowledged advances in identifying biomarkers, noting they outperform traditional phenomenological categories in identifying types of psychosis.[77][78] As a diagnostic biomarker, ΔFosB expression could be used to diagnose an addiction in humans, but this would require a brain biopsy and therefore isn't used in clinical practice.
Treatment
According to a review, "in order to be effective, all pharmacological or biologically based treatments for addiction need to be integrated into other established forms of addiction rehabilitation, such as cognitive behavioral therapy, individual and group psychotherapy, behavior-modification strategies, twelve-step programs, and residential treatment facilities."[7]
Behavioral therapy
This section needs expansion. You can help by adding to it. (March 2016) |
A meta-analytic review on the efficacy of various behavioral therapies for treating drug and behavioral addictions found that cognitive behavioral therapy (e.g., relapse prevention and contingency management), motivational interviewing, and a community reinforcement approach were effective interventions with moderate effect sizes.[79] Preclinical research using a rodent model of cue exposure therapy (CET) show that this type of treatment is more effective in adults compared to adolescents, however that adolescent outcomes can be improved by acute treatment at the time of (CET) with a dopamine 2 receptor agonist.[80]
Clinical and preclinical evidence indicate that consistent aerobic exercise, especially endurance exercise (e.g., marathon running), actually prevents the development of certain drug addictions and is an effective adjunct treatment for drug addiction, and for psychostimulant addiction in particular.[11][81][82][83][84] Consistent aerobic exercise magnitude-dependently (i.e., by duration and intensity) reduces drug addiction risk, which appears to occur through the reversal of drug induced addiction-related neuroplasticity.[11][82] One review noted that exercise may prevent the development of drug addiction by altering ΔFosB or c-Fos immunoreactivity in the striatum or other parts of the reward system.[84] Aerobic exercise decreases drug self-administration, reduces the likelihood of relapse, and induces opposite effects on striatal dopamine receptor D2 (DRD2) signaling (increased DRD2 density) to those induced by addictions to several drug classes (decreased DRD2 density).[11][82] Consequently, consistent aerobic exercise may lead to better treatment outcomes when used as an adjunct treatment for drug addiction.[11][82][83]
Medication
Alcohol addiction
This section needs more reliable medical references for verification or relies too heavily on primary sources. (April 2016) |  |
Alcohol, like opioids, can induce a severe state of physical dependence and produce withdrawal symptoms such as delirium tremens. Because of this, treatment for alcohol addiction usually involves a combined approach dealing with dependence and addiction simultaneously.
Pharmacological treatments for alcohol addiction include drugs like naltrexone (opioid antagonist), disulfiram, acamprosate, and topiramate.[85][86] Rather than substituting for alcohol, these drugs are intended to affect the desire to drink, either by directly reducing cravings as with acamprosate and topiramate, or by producing unpleasant effects when alcohol is consumed, as with disulfiram. These drugs can be effective if treatment is maintained, but compliance can be an issue as alcoholic patients often forget to take their medication, or discontinue use because of excessive side effects.[87][88] According to a Cochrane Collaboration review, the opioid antagonist naltrexone has short-term efficacy treating alcoholism, but evidence of longer term efficacy is lacking.[89]
Behavioral addictions
Behavioral addiction, process addiction,[90] or non-substance-related disorder[91] is a form of addiction that involves a compulsion to engage in a rewarding non-substance-related behavior – sometimes called a natural reward[13][11] – despite any negative consequences to the person's physical, mental, social or financial well-being.[92] In the brain's reward system, a gene transcription factor known as ΔFosB has been identified as a necessary common factor involved in both behavioral and drug addictions, which are associated with the same set of neural adaptations.[13][11][53]
Addiction canonically refers to substance abuse; however, the term's connotation has been expanded to include behaviors that may lead to a reward (such as gambling, eating, or shopping)[93] since the 1990s. Still, the framework to diagnose and categorize behavioral addiction is a controversial topic in the psychopathology field.[94][95]
Psychiatric and medical classifications
Diagnostic and Statistical Manual of Mental Disorders (DSM) recognized behavioral addictions for the first time in DSM-5 with gambling disorder, formerly pathological gambling, as the only non-substance-related disorder classified under the chapter of "Substance-Related and Addictive Disorders".[96] Internet gaming addiction was included in the appendix as a condition for further study.[97] Although "addiction" is commonly used to describe repetitive harmful behavior in nonmedical settings,[98] DSM-5 recommended the neutral term "disorder" instead of "addiction" under the clinical settings to avoid uncertain definition and potentially negative connotation.[99]
Similar to the changes in DSM-5, the eleventh revision of the International Classification of Diseases (ICD-11) introduced the category "Disorders due to substance use or addictive behaviours," based on the diagnostic framework of impaired control, repetitive harmful behavior, and continuation or escalation despite negative consequences.[100] The new sub-category "Disorders due to addictive behaviours" included gambling disorder (formerly under the habit and impulse disorders), gaming disorder (a new diagnosis), and two residual categories (other specified and unspecified) to raise attention among clinicians and the public and to facilitate further research.[100][101]
In 2019, the American Society of Addiction Medicine (ASAM) revised its definition of addiction including substance use and compulsive behaviors, stating: "addiction is a treatable, chronic medical disease involving complex interactions among brain circuits, genetics, the environment, and an individual’s life experiences."[102]
Other addictive behaviors which have received research attention but with insufficient or inconclusive evidence include pornography use disorder, compulsive buying disorder, social network use disorder, work addiction, exercise addiction, compulsive sexual behavior disorder, and food addiction.[99][103][104][105]
Treatment
Behavioral addiction is a treatable condition.[106] Treatment options include psychotherapy and psychopharmacotherapy (i.e., medications) or a combination of both. Cognitive behavioral therapy (CBT) is the most common form of psychotherapy used in treating behavioral addictions; it focuses on identifying patterns that trigger compulsive behavior and making lifestyle changes to promote healthier behaviors. Because cognitive behavioral therapy is considered a short-term therapy, the number of sessions for treatment normally ranges from five to twenty.[107] During the session, therapists will lead patients through the topics of identifying the issue, becoming aware of one's thoughts surrounding the issue, identifying any negative or false thinking, and reshaping said negative and false thinking. While CBT does not cure behavioral addiction, it does help with coping with the condition in a healthy way. Currently, there are no medications approved for treatment of behavioral addictions in general, but some medications used for treatment of drug addiction may also be beneficial with specific behavioral addictions.[26][108]
Another form of treatment is recreational therapy. A Certified Therapeutic Recreation Specialist (CTRS) uses leisure and recreation to help individuals recover from their injuries, ailments, or addictions. Therapeutic recreation can help an individual struggling with addiction to improve their self-esteem, confidence, motivation, resiliency, autonomy, enjoyment, and overall emotional state.[109][110]
Research
The classification and diagnostic framework of behavioral addictions under DSM-5 and ICD-11 has been a controversial subject among the clinical research field.[104] For example, this 2020 narrative review[103] considered ICD-11's guidelines to be adequate to include more behavioral addictions based on clinical relevance and empirical evidence, while this 2015 journal article questioned[111] the atheoretical and confirmatory research approaches on the accuracy of qualitative factors and criticized the lack of consideration of social elements and psychological processes.
A recent narrative review[112] in 2017 examined the existing literature for studies reporting associations between behavioral addictions (pathological gambling, problematic internet use, problematic online gaming, compulsive sexual behavior disorder, compulsive buying and exercise addiction) and psychiatric disorders. Overall, there is solid evidence for associations between behavioral addictions and mood disorder, anxiety disorder as well as substance use disorders. Associations between ADHD may be specific to problematic internet use and problematic online gaming. The authors also conclude that most of current research on the association between behavioral addictions and psychiatric disorders has several limitations: they are mostly cross-sectional, are not from representative samples, and are often based on small samples, among others. Especially more longitudinal studies are needed to establish the direction of causation, i.e. whether behavioral addictions are a cause or a consequence of psychiatric disorders.
Addiction and the reward system
ΔFosB, a gene transcription factor, has been identified as playing a critical role in the development of addictive states in both behavioral addictions and drug addictions.[13][11][53] Overexpression of ΔFosB in the nucleus accumbens is necessary and sufficient for many of the neural adaptations seen in drug addiction;[13] it has been implicated in addictions to alcohol, cannabinoids, cocaine, nicotine, phenylcyclidine, and substituted amphetamines[13][49][50][51] as well as addictions to natural rewards such as sex, exercise, and food.[11][53] A recent study also demonstrated a cross-sensitization between drug reward (amphetamine) and a natural reward (sex) that was mediated by ΔFosB.[113]
One of the major areas of study is the amygdala, a brain structure which involves emotional significance and associated learning. Research shows that dopaminergic projections from the ventral tegmental area facilitate a motivational or learned association to a specific behavior.[114] Dopamine neurons take a role in the learning and sustaining of many acquired behaviors. Research specific to Parkinson's disease has led to identifying the intracellular signaling pathways that underlie the immediate actions of dopamine. The most common mechanism of dopamine is to create addictive properties along with certain behaviors.[115] There are three stages to the dopamine reward system: bursts of dopamine, triggering of behavior, and further impact to the behavior. Once electronically signaled, possibly through the behavior, dopamine neurons let out a 'burst-fire' of elements to stimulate areas along fast transmitting pathways. The behavior response then perpetuates the striated neurons to further send stimuli. The fast firing of dopamine neurons can be monitored over time by evaluating the amount of extracellular concentrations of dopamine through micro dialysis and brain imaging. This monitoring can lead to a model in which one can see the multiplicity of triggering over a period of time.[116] Once the behavior is triggered, it is hard to work away from the dopamine reward system.
Behaviors like gambling have been linked to the newfound idea of the brain's capacity to anticipate rewards. The reward system can be triggered by early detectors of the behavior, and trigger dopamine neurons to begin stimulating behaviors. But in some cases, it can lead to many issues due to error, or reward-prediction errors. These errors can act as teaching signals to create a complex behavior task over time.[116]
See also
References
- ^ a b c d e f g h i j k l m n o p q r Nestler EJ (December 2013). "Cellular basis of memory for addiction". Dialogues in Clinical Neuroscience. 15 (4): 431–443. PMC 3898681. PMID 24459410.
Despite the importance of numerous psychosocial factors, at its core, drug addiction involves a biological process: the ability of repeated exposure to a drug of abuse to induce changes in a vulnerable brain that drive the compulsive seeking and taking of drugs, and loss of control over drug use, that define a state of addiction. ... A large body of literature has demonstrated that such ΔFosB induction in D1-type [nucleus accumbens] neurons increases an animal's sensitivity to drug as well as natural rewards and promotes drug self-administration, presumably through a process of positive reinforcement ... Another ΔFosB target is cFos: as ΔFosB accumulates with repeated drug exposure it represses c-Fos and contributes to the molecular switch whereby ΔFosB is selectively induced in the chronic drug-treated state.41 ... Moreover, there is increasing evidence that, despite a range of genetic risks for addiction across the population, exposure to sufficiently high doses of a drug for long periods of time can transform someone who has relatively lower genetic loading into an addict.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and Addictive Disorders". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 364–375. ISBN 978-0-07-148127-4.
- ^ a b Volkow ND, Koob GF, McLellan AT (January 2016). "Neurobiologic Advances from the Brain Disease Model of Addiction". New England Journal of Medicine. 374 (4): 363–371. doi:10.1056/NEJMra1511480. PMC 6135257. PMID 26816013.
Substance-use disorder: A diagnostic term in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) referring to recurrent use of alcohol or other drugs that causes clinically and functionally significant impairment, such as health problems, disability, and failure to meet major responsibilities at work, school, or home. Depending on the level of severity, this disorder is classified as mild, moderate, or severe.
Addiction: A term used to indicate the most severe, chronic stage of substance-use disorder, in which there is a substantial loss of self-control, as indicated by compulsive drug taking despite the desire to stop taking the drug. In the DSM-5, the term addiction is synonymous with the classification of severe substance-use disorder. - ^ a b Cite error: The named reference
Nestler Labs Glossarywas invoked but never defined (see the help page). - ^ Angres DH, Bettinardi-Angres K (October 2008). "The disease of addiction: origins, treatment, and recovery". Dis Mon. 54 (10): 696–721. doi:10.1016/j.disamonth.2008.07.002. PMID 18790142.
- ^ a b Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and Addictive Disorders". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 364–365, 375. ISBN 9780071481274.
The defining feature of addiction is compulsive, out-of-control drug use, despite negative consequences. ...
compulsive eating, shopping, gambling, and sex–so-called "natural addictions"– Indeed, addiction to both drugs and behavioral rewards may arise from similar dysregulation of the mesolimbic dopamine system. - ^ a b c Cite error: The named reference
Reward system and psychostimulantswas invoked but never defined (see the help page). - ^ [1][4][3][5][6][7]
- ^ American Society for Addiction Medicine (2012). "Definition of Addiction".
- ^ a b c d e f g h i j Ruffle JK (November 2014). "Molecular neurobiology of addiction: what's all the (Δ)FosB about?". Am. J. Drug Alcohol Abuse. 40 (6): 428–437. doi:10.3109/00952990.2014.933840. PMID 25083822.
The strong correlation between chronic drug exposure and ΔFosB provides novel opportunities for targeted therapies in addiction (118), and suggests methods to analyze their efficacy (119). Over the past two decades, research has progressed from identifying ΔFosB induction to investigating its subsequent action (38). It is likely that ΔFosB research will now progress into a new era – the use of ΔFosB as a biomarker. ...
Conclusions
ΔFosB is an essential transcription factor implicated in the molecular and behavioral pathways of addiction following repeated drug exposure. The formation of ΔFosB in multiple brain regions, and the molecular pathway leading to the formation of AP-1 complexes is well understood. The establishment of a functional purpose for ΔFosB has allowed further determination as to some of the key aspects of its molecular cascades, involving effectors such as GluR2 (87,88), Cdk5 (93) and NFkB (100). Moreover, many of these molecular changes identified are now directly linked to the structural, physiological and behavioral changes observed following chronic drug exposure (60,95,97,102). New frontiers of research investigating the molecular roles of ΔFosB have been opened by epigenetic studies, and recent advances have illustrated the role of ΔFosB acting on DNA and histones, truly as a molecular switch (34). As a consequence of our improved understanding of ΔFosB in addiction, it is possible to evaluate the addictive potential of current medications (119), as well as use it as a biomarker for assessing the efficacy of therapeutic interventions (121,122,124). Some of these proposed interventions have limitations (125) or are in their infancy (75). However, it is hoped that some of these preliminary findings may lead to innovative treatments, which are much needed in addiction. - ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an Olsen CM (December 2011). "Natural rewards, neuroplasticity, and non-drug addictions". Neuropharmacology. 61 (7): 1109–1122. doi:10.1016/j.neuropharm.2011.03.010. PMC 3139704. PMID 21459101.
Functional neuroimaging studies in humans have shown that gambling (Breiter et al, 2001), shopping (Knutson et al, 2007), orgasm (Komisaruk et al, 2004), playing video games (Koepp et al, 1998; Hoeft et al, 2008) and the sight of appetizing food (Wang et al, 2004a) activate many of the same brain regions (i.e., the mesocorticolimbic system and extended amygdala) as drugs of abuse (Volkow et al, 2004). ... Cross-sensitization is also bidirectional, as a history of amphetamine administration facilitates sexual behavior and enhances the associated increase in NAc DA ... As described for food reward, sexual experience can also lead to activation of plasticity-related signaling cascades. The transcription factor delta FosB is increased in the NAc, PFC, dorsal striatum, and VTA following repeated sexual behavior (Wallace et al., 2008; Pitchers et al., 2010b). This natural increase in delta FosB or viral overexpression of delta FosB within the NAc modulates sexual performance, and NAc blockade of delta FosB attenuates this behavior (Hedges et al, 2009; Pitchers et al., 2010b). Further, viral overexpression of delta FosB enhances the conditioned place preference for an environment paired with sexual experience (Hedges et al., 2009). ... In some people, there is a transition from "normal" to compulsive engagement in natural rewards (such as food or sex), a condition that some have termed behavioral or non-drug addictions (Holden, 2001; Grant et al., 2006a). ... In humans, the role of dopamine signaling in incentive-sensitization processes has recently been highlighted by the observation of a dopamine dysregulation syndrome in some patients taking dopaminergic drugs. This syndrome is characterized by a medication-induced increase in (or compulsive) engagement in non-drug rewards such as gambling, shopping, or sex (Evans et al, 2006; Aiken, 2007; Lader, 2008)."
Table 1" Cite error: The named reference "Natural and drug addictions" was defined multiple times with different content (see the help page). - ^ a b c d e f Biliński P, Wojtyła A, Kapka-Skrzypczak L, Chwedorowicz R, Cyranka M, Studziński T (2012). "Epigenetic regulation in drug addiction". Ann. Agric. Environ. Med. 19 (3): 491–496. PMID 23020045.
For these reasons, ΔFosB is considered a primary and causative transcription factor in creating new neural connections in the reward centre, prefrontal cortex, and other regions of the limbic system. This is reflected in the increased, stable and long-lasting level of sensitivity to cocaine and other drugs, and tendency to relapse even after long periods of abstinence. These newly constructed networks function very efficiently via new pathways as soon as drugs of abuse are further taken ... In this way, the induction of CDK5 gene expression occurs together with suppression of the G9A gene coding for dimethyltransferase acting on the histone H3. A feedback mechanism can be observed in the regulation of these 2 crucial factors that determine the adaptive epigenetic response to cocaine. This depends on ΔFosB inhibiting G9a gene expression, i.e. H3K9me2 synthesis which in turn inhibits transcription factors for ΔFosB. For this reason, the observed hyper-expression of G9a, which ensures high levels of the dimethylated form of histone H3, eliminates the neuronal structural and plasticity effects caused by cocaine by means of this feedback which blocks ΔFosB transcription
- ^ a b c d e f g h i j k l m n o p q Robison AJ, Nestler EJ (November 2011). "Transcriptional and epigenetic mechanisms of addiction". Nat. Rev. Neurosci. 12 (11): 623–637. doi:10.1038/nrn3111. PMC 3272277. PMID 21989194.
ΔFosB has been linked directly to several addiction-related behaviors ... Importantly, genetic or viral overexpression of ΔJunD, a dominant negative mutant of JunD which antagonizes ΔFosB- and other AP-1-mediated transcriptional activity, in the NAc or OFC blocks these key effects of drug exposure14,22–24. This indicates that ΔFosB is both necessary and sufficient for many of the changes wrought in the brain by chronic drug exposure. ΔFosB is also induced in D1-type NAc MSNs by chronic consumption of several natural rewards, including sucrose, high fat food, sex, wheel running, where it promotes that consumption14,26–30. This implicates ΔFosB in the regulation of natural rewards under normal conditions and perhaps during pathological addictive-like states.
Cite error: The named reference "Nestler" was defined multiple times with different content (see the help page). - ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 1: Basic Principles of Neuropharmacology". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 4. ISBN 9780071481274.
Drug abuse and addiction exact an astoundingly high financial and human toll on society through direct adverse effects, such as lung cancer and hepatic cirrhosis, and indirect adverse effects—for example, accidents and AIDS—on health and productivity.
- ^ "Epidemiology of Substance Use Disorders". Hum. Genet. 131 (6): 779–789. June 2012. doi:10.1007/s00439-012-1168-0. PMC 4408274. PMID 22543841.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Cite error: The named reference
ABAMwas invoked but never defined (see the help page). - ^ Morse RM, Flavin DK (August 1992). "The definition of alcoholism. The Joint Committee of the National Council on Alcoholism and Drug Dependence and the American Society of Addiction Medicine to Study the Definition and Criteria for the Diagnosis of Alcoholism". JAMA. 268 (8): 1012–4. doi:10.1001/jama.1992.03490080086030. PMID 1501306.
- ^ Marlatt GA, Baer JS, Donovan DM, Kivlahan DR (1988). "Addictive behaviors: etiology and treatment". Annu Rev Psychol. 39: 223–52. doi:10.1146/annurev.ps.39.020188.001255. PMID 3278676.
- ^ a b American Psychiatric Association (2013). "Substance-Related and Addictive Disorders" (PDF). American Psychiatric Publishing. pp. 1–2. Retrieved 10 July 2015.
Additionally, the diagnosis of dependence caused much confusion. Most people link dependence with "addiction" when in fact dependence can be a normal body response to a substance.
- ^ Washburn DA (2016). "The Stroop effect at 80: The competition between stimulus control and cognitive control". J Exp Anal Behav. 105 (1): 3–13. doi:10.1002/jeab.194. PMID 26781048.
Today, arguably more than at any time in history, the constructs of attention, executive functioning, and cognitive control seem to be pervasive and preeminent in research and theory. Even within the cognitive framework, however, there has long been an understanding that behavior is multiply determined, and that many responses are relatively automatic, unattended, contention-scheduled, and habitual. Indeed, the cognitive flexibility, response inhibition, and self-regulation that appear to be hallmarks of cognitive control are noteworthy only in contrast to responses that are relatively rigid, associative, and involuntary.
- ^ Diamond A (2013). "Executive functions". Annu Rev Psychol. 64: 135–168. doi:10.1146/annurev-psych-113011-143750. PMC 4084861. PMID 23020641.
Core EFs are inhibition [response inhibition (self-control—resisting temptations and resisting acting impulsively) and interference control (selective attention and cognitive inhibition)], working memory, and cognitive flexibility (including creatively thinking "outside the box," seeing anything from different perspectives, and quickly and flexibly adapting to changed circumstances). ... EFs and prefrontal cortex are the first to suffer, and suffer disproportionately, if something is not right in your life. They suffer first, and most, if you are stressed (Arnsten 1998, Liston et al. 2009, Oaten & Cheng 2005), sad (Hirt et al. 2008, von Hecker & Meiser 2005), lonely (Baumeister et al. 2002, Cacioppo & Patrick 2008, Campbell et al. 2006, Tun et al. 2012), sleep deprived (Barnes et al. 2012, Huang et al. 2007), or not physically fit (Best 2010, Chaddock et al. 2011, Hillman et al. 2008). Any of these can cause you to appear to have a disorder of EFs, such as ADHD, when you do not. You can see the deleterious effects of stress, sadness, loneliness, and lack of physical health or fitness at the physiological and neuroanatomical level in prefrontal cortex and at the behavioral level in worse EFs (poorer reasoning and problem solving, forgetting things, and impaired ability to exercise discipline and self-control). ...
EFs can be improved (Diamond & Lee 2011, Klingberg 2010). ... At any age across the life cycle EFs can be improved, including in the elderly and in infants. There has been much work with excellent results on improving EFs in the elderly by improving physical fitness (Erickson & Kramer 2009, Voss et al. 2011) ... Inhibitory control (one of the core EFs) involves being able to control one's attention, behavior, thoughts, and/or emotions to override a strong internal predisposition or external lure, and instead do what's more appropriate or needed. Without inhibitory control we would be at the mercy of impulses, old habits of thought or action (conditioned responses), and/or stimuli in the environment that pull us this way or that. Thus, inhibitory control makes it possible for us to change and for us to choose how we react and how we behave rather than being unthinking creatures of habit. It doesn't make it easy. Indeed, we usually are creatures of habit and our behavior is under the control of environmental stimuli far more than we usually realize, but having the ability to exercise inhibitory control creates the possibility of change and choice. ... The subthalamic nucleus appears to play a critical role in preventing such impulsive or premature responding (Frank 2006). - ^ a b Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 13: Higher Cognitive Function and Behavioral Control". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 313–321. ISBN 9780071481274.
• Executive function, the cognitive control of behavior, depends on the prefrontal cortex, which is highly developed in higher primates and especially humans.
• Working memory is a short-term, capacity-limited cognitive buffer that stores information and permits its manipulation to guide decision-making and behavior. ...
These diverse inputs and back projections to both cortical and subcortical structures put the prefrontal cortex in a position to exert what is often called "top-down" control or cognitive control of behavior. ... The prefrontal cortex receives inputs not only from other cortical regions, including association cortex, but also, via the thalamus, inputs from subcortical structures subserving emotion and motivation, such as the amygdala (Chapter 14) and ventral striatum (or nucleus accumbens; Chapter 15). ...
In conditions in which prepotent responses tend to dominate behavior, such as in drug addiction, where drug cues can elicit drug seeking (Chapter 15), or in attention deficit hyperactivity disorder (ADHD; described below), significant negative consequences can result. ... ADHD can be conceptualized as a disorder of executive function; specifically, ADHD is characterized by reduced ability to exert and maintain cognitive control of behavior. Compared with healthy individuals, those with ADHD have diminished ability to suppress inappropriate prepotent responses to stimuli (impaired response inhibition) and diminished ability to inhibit responses to irrelevant stimuli (impaired interference suppression). ... Functional neuroimaging in humans demonstrates activation of the prefrontal cortex and caudate nucleus (part of the striatum) in tasks that demand inhibitory control of behavior. Subjects with ADHD exhibit less activation of the medial prefrontal cortex than healthy controls even when they succeed in such tasks and utilize different circuits. ... Early results with structural MRI show thinning of the cerebral cortex in ADHD subjects compared with age-matched controls in prefrontal cortex and posterior parietal cortex, areas involved in working memory and attention. - ^ a b Cite error: The named reference
Systematic review - yet another DSM failwas invoked but never defined (see the help page). - ^ a b c d e Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM (February 2013). "Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator". The Journal of Neuroscience. 33 (8): 3434–3442. doi:10.1523/JNEUROSCI.4881-12.2013. PMC 3865508. PMID 23426671.
Drugs of abuse induce neuroplasticity in the natural reward pathway, specifically the nucleus accumbens (NAc), thereby causing development and expression of addictive behavior. ... Together, these findings demonstrate that drugs of abuse and natural reward behaviors act on common molecular and cellular mechanisms of plasticity that control vulnerability to drug addiction, and that this increased vulnerability is mediated by ΔFosB and its downstream transcriptional targets. ... Sexual behavior is highly rewarding (Tenk et al., 2009), and sexual experience causes sensitized drug-related behaviors, including cross-sensitization to amphetamine (Amph)-induced locomotor activity (Bradley and Meisel, 2001; Pitchers et al., 2010a) and enhanced Amph reward (Pitchers et al., 2010a). Moreover, sexual experience induces neural plasticity in the NAc similar to that induced by psychostimulant exposure, including increased dendritic spine density (Meisel and Mullins, 2006; Pitchers et al., 2010a), altered glutamate receptor trafficking, and decreased synaptic strength in prefrontal cortex-responding NAc shell neurons (Pitchers et al., 2012). Finally, periods of abstinence from sexual experience were found to be critical for enhanced Amph reward, NAc spinogenesis (Pitchers et al., 2010a), and glutamate receptor trafficking (Pitchers et al., 2012). These findings suggest that natural and drug reward experiences share common mechanisms of neural plasticity
- ^ a b c d e Beloate LN, Weems PW, Casey GR, Webb IC, Coolen LM (February 2016). "Nucleus accumbens NMDA receptor activation regulates amphetamine cross-sensitization and deltaFosB expression following sexual experience in male rats". Neuropharmacology. 101: 154–164. doi:10.1016/j.neuropharm.2015.09.023. PMID 26391065.
- ^ a b c Grant JE, Potenza MN, Weinstein A, Gorelick DA (September 2010). "Introduction to behavioral addictions". Am. J. Drug Alcohol Abuse. 36 (5): 233–241. doi:10.3109/00952990.2010.491884. PMC 3164585. PMID 20560821.
Naltrexone, a mu-opioid receptor antagonist approved by the US Food and Drug Administration for the treatment of alcoholism and opioid dependence, has shown efficacy in controlled clinical trials for the treatment of pathological gambling and kleptomania (76–79), and promise in uncontrolled studies of compulsive buying (80), compulsive sexual behavior (81), internet addiction (82), and pathologic skin picking (83). ... Topiramate, an anti-convulsant which blocks the AMPA subtype of glutamate receptor (among other actions), has shown promise in open-label studies of pathological gambling, compulsive buying, and compulsive skin picking (85), as well as efficacy in reducing alcohol (86), cigarette (87), and cocaine (88) use. N-acetyl cysteine, an amino acid that restores extracellular glutamate concentration in the nucleus accumbens, reduced gambling urges and behavior in one study of pathological gamblers (89), and reduces cocaine craving (90) and cocaine use (91) in cocaine addicts. These studies suggest that glutamatergic modulation of dopaminergic tone in the nucleus accumbens may be a mechanism common to behavioral addiction and substance use disorders (92).
- ^ a b c d e f Vassoler FM, Sadri-Vakili G (2014). "Mechanisms of transgenerational inheritance of addictive-like behaviors". Neuroscience. 264: 198–206. doi:10.1016/j.neuroscience.2013.07.064. PMC 3872494. PMID 23920159.
However, the components that are responsible for the heritability of characteristics that make an individual more susceptible to drug addiction in humans remain largely unknown given that patterns of inheritance cannot be explained by simple genetic mechanisms (Cloninger et al., 1981; Schuckit et al., 1972). The environment also plays a large role in the development of addiction as evidenced by great societal variability in drug use patterns between countries and across time (UNODC, 2012). Therefore, both genetics and the environment contribute to an individual's vulnerability to become addicted following an initial exposure to drugs of abuse. ...
The evidence presented here demonstrates that rapid environmental adaptation occurs following exposure to a number of stimuli. Epigenetic mechanisms represent the key components by which the environment can influence genetics, and they provide the missing link between genetic heritability and environmental influences on the behavioral and physiological phenotypes of the offspring. - ^ Spear LP (June 2000). "The adolescent brain and age-related behavioral manifestations". Neuroscience and Biobehavioral Reviews. 24 (4): 417–463. doi:10.1016/s0149-7634(00)00014-2. PMID 10817843.
- ^ Catalano RF, Hawkins JD, Wells EA, Miller J, Brewer D (1990). "Evaluation of the effectiveness of adolescent drug abuse treatment, assessment of risks for relapse, and promising approaches for relapse prevention". The International Journal of the Addictions. 25 (9A–10A): 1085–140. PMID 2131328.
- ^ Perepletchikova F, Krystal JH, Kaufman J (November 2008). "Practitioner review: adolescent alcohol use disorders: assessment and treatment issues". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 49 (11): 1131–1154. doi:10.1111/j.1469-7610.2008.01934.x. PMC 4113213. PMID 19017028.
- ^ http://alcoholrehab.com/drug-addiction/age-and-substance-abuse/
- ^ a b Kendler KS, Neale MC, Heath AC, Kessler RC, Eaves LJ (May 1994). "A twin-family study of alcoholism in women". Am J Psychiatry. 151 (5): 707–15. PMID 8166312.
- ^ Clarke TK, Crist RC, Kampman KM, Dackis CA, Pettinati HM, O'Brien CP, Oslin DW, Ferraro TN, Lohoff FW, Berrettini WH (2013). "Low frequency genetic variants in the μ-opioid receptor (OPRM1) affect risk for addiction to heroin and cocaine". Neuroscience Letters. 542: 71–5. doi:10.1016/j.neulet.2013.02.018. PMC 3640707. PMID 23454283.
- ^ Hall, F. Scott; Drgonova, Jana; Jain, Siddharth; Uhl, George R. (December 2013). "Implications of genome wide association studies for addiction: Are our a priori assumptions all wrong?". Pharmacology & Therapeutics. 140 (3): 267–279. doi:10.1016/j.pharmthera.2013.07.006.
- ^ a b "Adverse Childhood Experiences". samhsa.gov. Rockville, Maryland, United States: Substance Abuse and Mental Health Services Administration. Retrieved 26 September 2016.
- ^ Abuse, National Institute on Drug. "What are risk factors and protective factors?". Retrieved 2016-12-19.
- ^ SAMSHA. "Risk and Protective Factors". https://www.samhsa.gov/capt/practicing-effective-prevention/prevention-behavioral-health/risk-protective-factors. Substance Abuse and Mental Health Administration. Retrieved 2016-12-19.
{{cite web}}: External link in|website= - ^ "Infographic – Risk Factors of Addiction | Recovery Research Institute". www.recoveryanswers.org. Retrieved 2016-12-19.
- ^ "Drug addiction Risk factors - Mayo Clinic". www.mayoclinic.org. Retrieved 2016-12-19.
- ^ Abuse, National Institute on Drug. "What are risk factors and protective factors?". Retrieved 2016-12-19.
- ^ a b c Yuan TF, Li A, Sun X, Ouyang H, Campos C, Rocha NB, Arias-Carrión O, Machado S, Hou G, So KF (2015). "Transgenerational Inheritance of Paternal Neurobehavioral Phenotypes: Stress, Addiction, Ageing and Metabolism". Mol. Neurobiol. doi:10.1007/s12035-015-9526-2. PMID 26572641.
- ^ a b c Renthal W, Nestler EJ (September 2009). "Chromatin regulation in drug addiction and depression". Dialogues in Clinical Neuroscience. 11 (3): 257–268. doi:10.31887/DCNS.2009.11.3/wrenthal. PMC 2834246. PMID 19877494.
[Psychostimulants] increase cAMP levels in striatum, which activates protein kinase A (PKA) and leads to phosphorylation of its targets. This includes the cAMP response element binding protein (CREB), the phosphorylation of which induces its association with the histone acetyltransferase, CREB binding protein (CBP) to acetylate histones and facilitate gene activation. This is known to occur on many genes including fosB and c-fos in response to psychostimulant exposure. ΔFosB is also upregulated by chronic psychostimulant treatments, and is known to activate certain genes (eg, cdk5) and repress others (eg, c-fos) where it recruits HDAC1 as a corepressor. ... Chronic exposure to psychostimulants increases glutamatergic [signaling] from the prefrontal cortex to the NAc. Glutamatergic signaling elevates Ca2+ levels in NAc postsynaptic elements where it activates CaMK (calcium/calmodulin protein kinases) signaling, which, in addition to phosphorylating CREB, also phosphorylates HDAC5.
Figure 2: Psychostimulant-induced signaling events - ^ Broussard JI (January 2012). "Co-transmission of dopamine and glutamate". The Journal of General Physiology. 139 (1): 93–96. doi:10.1085/jgp.201110659. PMC 3250102. PMID 22200950.
Coincident and convergent input often induces plasticity on a postsynaptic neuron. The NAc integrates processed information about the environment from basolateral amygdala, hippocampus, and prefrontal cortex (PFC), as well as projections from midbrain dopamine neurons. Previous studies have demonstrated how dopamine modulates this integrative process. For example, high frequency stimulation potentiates hippocampal inputs to the NAc while simultaneously depressing PFC synapses (Goto and Grace, 2005). The converse was also shown to be true; stimulation at PFC potentiates PFC–NAc synapses but depresses hippocampal–NAc synapses. In light of the new functional evidence of midbrain dopamine/glutamate co-transmission (references above), new experiments of NAc function will have to test whether midbrain glutamatergic inputs bias or filter either limbic or cortical inputs to guide goal-directed behavior.
- ^ Kanehisa Laboratories (10 October 2014). "Amphetamine – Homo sapiens (human)". KEGG Pathway. Retrieved 31 October 2014.
Most addictive drugs increase extracellular concentrations of dopamine (DA) in nucleus accumbens (NAc) and medial prefrontal cortex (mPFC), projection areas of mesocorticolimbic DA neurons and key components of the "brain reward circuit". Amphetamine achieves this elevation in extracellular levels of DA by promoting efflux from synaptic terminals. ... Chronic exposure to amphetamine induces a unique transcription factor delta FosB, which plays an essential role in long-term adaptive changes in the brain.
- ^ Cadet JL, Brannock C, Jayanthi S, Krasnova IN (2015). "Transcriptional and epigenetic substrates of methamphetamine addiction and withdrawal: evidence from a long-access self-administration model in the rat". Molecular Neurobiology. 51 (2): 696–717 (Figure 1). doi:10.1007/s12035-014-8776-8. PMC 4359351. PMID 24939695.
- ^ a b c Robison AJ, Nestler EJ (November 2011). "Transcriptional and epigenetic mechanisms of addiction". Nature Reviews Neuroscience. 12 (11): 623–637. doi:10.1038/nrn3111. PMC 3272277. PMID 21989194.
ΔFosB serves as one of the master control proteins governing this structural plasticity. ... ΔFosB also represses G9a expression, leading to reduced repressive histone methylation at the cdk5 gene. The net result is gene activation and increased CDK5 expression. ... In contrast, ΔFosB binds to the c-fos gene and recruits several co-repressors, including HDAC1 (histone deacetylase 1) and SIRT 1 (sirtuin 1). ... The net result is c-fos gene repression.
Figure 4: Epigenetic basis of drug regulation of gene expression - ^ a b c d Nestler EJ (December 2012). "Transcriptional mechanisms of drug addiction". Clinical Psychopharmacology and Neuroscience. 10 (3): 136–143. doi:10.9758/cpn.2012.10.3.136. PMC 3569166. PMID 23430970.
The 35-37 kD ΔFosB isoforms accumulate with chronic drug exposure due to their extraordinarily long half-lives. ... As a result of its stability, the ΔFosB protein persists in neurons for at least several weeks after cessation of drug exposure. ... ΔFosB overexpression in nucleus accumbens induces NFκB ... In contrast, the ability of ΔFosB to repress the c-Fos gene occurs in concert with the recruitment of a histone deacetylase and presumably several other repressive proteins such as a repressive histone methyltransferase
- ^ Nestler EJ (October 2008). "Transcriptional mechanisms of addiction: Role of ΔFosB". Philosophical Transactions of the Royal Society B: Biological Sciences. 363 (1507): 3245–3255. doi:10.1098/rstb.2008.0067. PMC 2607320. PMID 18640924.
Recent evidence has shown that ΔFosB also represses the c-fos gene that helps create the molecular switch—from the induction of several short-lived Fos family proteins after acute drug exposure to the predominant accumulation of ΔFosB after chronic drug exposure
- ^ a b c Hyman SE, Malenka RC, Nestler EJ (2006). "Neural mechanisms of addiction: the role of reward-related learning and memory". Annu. Rev. Neurosci. 29: 565–598. doi:10.1146/annurev.neuro.29.051605.113009. PMID 16776597. Cite error: The named reference "Nestler, Hyman, and Malenka 2" was defined multiple times with different content (see the help page).
- ^ a b Steiner H, Van Waes V (January 2013). "Addiction-related gene regulation: risks of exposure to cognitive enhancers vs. other psychostimulants". Prog. Neurobiol. 100: 60–80. doi:10.1016/j.pneurobio.2012.10.001. PMC 3525776. PMID 23085425. Cite error: The named reference "Addiction genetics" was defined multiple times with different content (see the help page).
- ^ a b Kanehisa Laboratories (2 August 2013). "Alcoholism – Homo sapiens (human)". KEGG Pathway. Retrieved 10 April 2014. Cite error: The named reference "Alcoholism ΔFosB" was defined multiple times with different content (see the help page).
- ^ Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P (February 2009). "Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens". Proc. Natl. Acad. Sci. U.S.A. 106 (8): 2915–2920. doi:10.1073/pnas.0813179106. PMC 2650365. PMID 19202072.
- ^ a b c d e f g Blum K, Werner T, Carnes S, Carnes P, Bowirrat A, Giordano J, Oscar-Berman M, Gold M (2012). "Sex, drugs, and rock 'n' roll: hypothesizing common mesolimbic activation as a function of reward gene polymorphisms". J. Psychoactive Drugs. 44 (1): 38–55. doi:10.1080/02791072.2012.662112. PMC 4040958. PMID 22641964.
It has been found that deltaFosB gene in the NAc is critical for reinforcing effects of sexual reward. Pitchers and colleagues (2010) reported that sexual experience was shown to cause DeltaFosB accumulation in several limbic brain regions including the NAc, medial pre-frontal cortex, VTA, caudate, and putamen, but not the medial preoptic nucleus. Next, the induction of c-Fos, a downstream (repressed) target of DeltaFosB, was measured in sexually experienced and naive animals. The number of mating-induced c-Fos-IR cells was significantly decreased in sexually experienced animals compared to sexually naive controls. Finally, DeltaFosB levels and its activity in the NAc were manipulated using viral-mediated gene transfer to study its potential role in mediating sexual experience and experience-induced facilitation of sexual performance. Animals with DeltaFosB overexpression displayed enhanced facilitation of sexual performance with sexual experience relative to controls. In contrast, the expression of DeltaJunD, a dominant-negative binding partner of DeltaFosB, attenuated sexual experience-induced facilitation of sexual performance, and stunted long-term maintenance of facilitation compared to DeltaFosB overexpressing group. Together, these findings support a critical role for DeltaFosB expression in the NAc in the reinforcing effects of sexual behavior and sexual experience-induced facilitation of sexual performance. ... both drug addiction and sexual addiction represent pathological forms of neuroplasticity along with the emergence of aberrant behaviors involving a cascade of neurochemical changes mainly in the brain's rewarding circuitry.
Cite error: The named reference "ΔFosB reward" was defined multiple times with different content (see the help page). - ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and addictive disorders". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 384–385. ISBN 9780071481274.
- ^ Salamone, J.D. (1992). "Complex motor and sensorimotor function of striatal and accumbens dopamine: Involvement in instrumental behavior processes". Psychopharmacology. 107: 160–174. doi:10.1007/bf02245133.
- ^ Kauer, J.A.; R.C. Malenka (2007). "Synaptic plasticity and addiction". Nature Reviews Neuroscience. 8 (11): 844–858. doi:10.1038/nrn2234. PMID 17948030.
- ^ Witten, I; S.-C. Lin; M Brodsky (2010). "Cholinergic interneurons control local circuit activity and cocaine conditioning". Science. 330: 1677–1681. doi:10.1126/science.1193771. PMC 3142356. PMID 21164015.
- ^ a b Nestler EJ, Barrot M, Self DW (September 2001). "DeltaFosB: a sustained molecular switch for addiction". Proc. Natl. Acad. Sci. U.S.A. 98 (20): 11042–11046. doi:10.1073/pnas.191352698. PMC 58680. PMID 11572966.
Although the ΔFosB signal is relatively long-lived, it is not permanent. ΔFosB degrades gradually and can no longer be detected in brain after 1–2 months of drug withdrawal ... Indeed, ΔFosB is the longest-lived adaptation known to occur in adult brain, not only in response to drugs of abuse, but to any other perturbation (that doesn't involve lesions) as well.
- ^ a b Jones S, Bonci A (2005). "Synaptic plasticity and drug addiction". Current Opinion in Pharmacology. 5 (1): 20–5. doi:10.1016/j.coph.2004.08.011. PMID 15661621.
- ^ a b Eisch AJ, Harburg GC (2006). "Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology". Hippocampus. 16 (3): 271–86. doi:10.1002/hipo.20161. PMID 16411230.
- ^ Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. p. 596. ISBN 0-443-07145-4.
- ^ Kourrich S, Rothwell PE, Klug JR, Thomas MJ (2007). "Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens". J. Neurosci. 27 (30): 7921–8. doi:10.1523/JNEUROSCI.1859-07.2007. PMID 17652583.
- ^ a b Kalivas PW, Volkow ND (August 2005). "The neural basis of addiction: a pathology of motivation and choice". The American Journal of Psychiatry. 162 (8): 1403–13. doi:10.1176/appi.ajp.162.8.1403. PMID 16055761.
- ^ a b Floresco SB, Ghods-Sharifi S (February 2007). "Amygdala-prefrontal cortical circuitry regulates effort-based decision making". Cerebral Cortex. 17 (2): 251–60. doi:10.1093/cercor/bhj143. PMID 16495432.
- ^ Perry CJ, Zbukvic I, Kim JH, Lawrence AJ (October 2014). "Role of cues and contexts on drug-seeking behaviour". British Journal of Pharmacology. 171 (20): 4636–72. doi:10.1111/bph.12735. PMC 4209936. PMID 24749941.
- ^ a b c Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F (2007). "Dopamine in drug abuse and addiction: results of imaging studies and treatment implications". Arch. Neurol. 64 (11): 1575–9. doi:10.1001/archneur.64.11.1575. PMID 17998440.
- ^ "Drugs, Brains, and Behavior: The Science of Addiction". National Institute on Drug Abuse.
- ^ "Understanding Drug Abuse and Addiction". National Institute on Drug Abuse. November 2012.
- ^ a b c Nestler EJ (October 2008). "Review. Transcriptional mechanisms of addiction: role of DeltaFosB". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 363 (1507): 3245–3255. doi:10.1098/rstb.2008.0067. PMC 2607320. PMID 18640924.
Recent evidence has shown that ΔFosB also represses the c-fos gene that helps create the molecular switch—from the induction of several short-lived Fos family proteins after acute drug exposure to the predominant accumulation of ΔFosB after chronic drug exposure—cited earlier (Renthal et al. in press). The mechanism responsible for ΔFosB repression of c-fos expression is complex and is covered below. ...
Examples of validated targets for ΔFosB in nucleus accumbens ... GluR2 ... dynorphin ... Cdk5 ... NFκB ... c-Fos
Table 3 - ^ a b c d e Berridge KC (April 2012). "From prediction error to incentive salience: mesolimbic computation of reward motivation". Eur. J. Neurosci. 35 (7): 1124–1143. doi:10.1111/j.1460-9568.2012.07990.x. PMC 3325516. PMID 22487042.
Here I discuss how mesocorticolimbic mechanisms generate the motivation component of incentive salience. Incentive salience takes Pavlovian learning and memory as one input and as an equally important input takes neurobiological state factors (e.g. drug states, appetite states, satiety states) that can vary independently of learning. Neurobiological state changes can produce unlearned fluctuations or even reversals in the ability of a previously learned reward cue to trigger motivation. Such fluctuations in cue-triggered motivation can dramatically depart from all previously learned values about the associated reward outcome. ... Associative learning and prediction are important contributors to motivation for rewards. Learning gives incentive value to arbitrary cues such as a Pavlovian conditioned stimulus (CS) that is associated with a reward (unconditioned stimulus or UCS). Learned cues for reward are often potent triggers of desires. For example, learned cues can trigger normal appetites in everyone, and can sometimes trigger compulsive urges and relapse in addicts.
Cue-triggered 'wanting' for the UCS
A brief CS encounter (or brief UCS encounter) often primes a pulse of elevated motivation to obtain and consume more reward UCS. This is a signature feature of incentive salience.
Cue as attractive motivational magnets
When a Pavlovian CS+ is attributed with incentive salience it not only triggers 'wanting' for its UCS, but often the cue itself becomes highly attractive – even to an irrational degree. This cue attraction is another signature feature of incentive salience ... Two recognizable features of incentive salience are often visible that can be used in neuroscience experiments: (i) UCS-directed 'wanting' – CS-triggered pulses of intensified 'wanting' for the UCS reward; and (ii) CS-directed 'wanting' – motivated attraction to the Pavlovian cue, which makes the arbitrary CS stimulus into a motivational magnet. - ^ a b Malenka RC, Nestler EJ, Hyman SE (2009). Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 147–148, 366–367, 375–376. ISBN 978-0-07-148127-4.
VTA DA neurons play a critical role in motivation, reward-related behavior (Chapter 15), attention, and multiple forms of memory. This organization of the DA system, wide projection from a limited number of cell bodies, permits coordinated responses to potent new rewards. Thus, acting in diverse terminal fields, dopamine confers motivational salience ("wanting") on the reward itself or associated cues (nucleus accumbens shell region), updates the value placed on different goals in light of this new experience (orbital prefrontal cortex), helps consolidate multiple forms of memory (amygdala and hippocampus), and encodes new motor programs that will facilitate obtaining this reward in the future (nucleus accumbens core region and dorsal striatum). In this example, dopamine modulates the processing of sensorimotor information in diverse neural circuits to maximize the ability of the organism to obtain future rewards. ...
The brain reward circuitry that is targeted by addictive drugs normally mediates the pleasure and strengthening of behaviors associated with natural reinforcers, such as food, water, and sexual contact. Dopamine neurons in the VTA are activated by food and water, and dopamine release in the NAc is stimulated by the presence of natural reinforcers, such as food, water, or a sexual partner. ...
The NAc and VTA are central components of the circuitry underlying reward and memory of reward. As previously mentioned, the activity of dopaminergic neurons in the VTA appears to be linked to reward prediction. The NAc is involved in learning associated with reinforcement and the modulation of motoric responses to stimuli that satisfy internal homeostatic needs. The shell of the NAc appears to be particularly important to initial drug actions within reward circuitry; addictive drugs appear to have a greater effect on dopamine release in the shell than in the core of the NAc. ... If motivational drive is described in terms of wanting, and hedonic evaluation in terms of liking, it appears that wanting can be dissociated from liking and that dopamine may influence these phenomena differently. Differences between wanting and liking are confirmed in reports by human addicts, who state that their desire for drugs (wanting) increases with continued use even when pleasure (liking) decreases because of tolerance. - ^ a b c Edwards S (2016). "Reinforcement principles for addiction medicine; from recreational drug use to psychiatric disorder". Prog. Brain Res. 223: 63–76. doi:10.1016/bs.pbr.2015.07.005. PMID 26806771.
An important dimension of reinforcement highly relevant to the addiction process (and particularly relapse) is secondary reinforcement (Stewart, 1992). Secondary reinforcers (in many cases also considered conditioned reinforcers) likely drive the majority of reinforcement processes in humans. In the specific case of drug addition, cues and contexts that are intimately and repeatedly associated with drug use will often themselves become reinforcing ... A fundamental piece of Robinson and Berridge's incentive-sensitization theory of addiction posits that the incentive value or attractive nature of such secondary reinforcement processes, in addition to the primary reinforcers themselves, may persist and even become sensitized over time in league with the development of drug addiction (Robinson and Berridge, 1993).
- ^ Traynor J (March 2012). "μ-Opioid receptors and regulators of G protein signaling (RGS) proteins: from a symposium on new concepts in mu-opioid pharmacology". Drug Alcohol Depend. 121 (3): 173–80. doi:10.1016/j.drugalcdep.2011.10.027. PMC 3288798. PMID 22129844.
- ^ Torres G, Horowitz JM (1999). "Drugs of abuse and brain gene expression". Psychosom Med. 61 (5): 630–50. doi:10.1097/00006842-199909000-00007. PMID 10511013.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and Addictive Disorders". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 364–368. ISBN 9780071481274.
The official diagnosis of drug addiction by the Diagnostic and Statistic Manual of Mental Disorders (2000), which makes distinctions between drug use, abuse, and substance dependence, is flawed. First, diagnosis of drug use versus abuse can be arbitrary and reflect cultural norms, not medical phenomena. Second, the term substance dependence implies that dependence is the primary pharmacologic phenomenon underlying addiction, which is likely not true, as tolerance, sensitization, and learning and memory also play central roles. It is ironic and unfortunate that the Manual avoids use of the term addiction, which provides the best description of the clinical syndrome.
- ^ Thomas Insel. "Transforming Diagnosis". National Institute of Mental Health. Retrieved 17 June 2015.
- ^ "Biomarkers outperform symptoms in parsing psychosis subgroups". National Institutes of Health. 8 December 2015. Retrieved 20 May 2016.
- ^ Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, Keshavan MS, Tamminga CA (2016). "Identification of Distinct Psychosis Biotypes Using Brain-Based Biomarkers". Am J Psychiatry. 173 (4): 373–84. doi:10.1176/appi.ajp.2015.14091200. PMID 26651391.
- ^ Walter M, Dürsteler KM, Petitjean SA, Wiesbeck GA, Euler S, Sollberger D, Lang UE, Vogel M (2015). "[Psychosocial Treatment of Addictive Disorders—An Overview of Psychotherapeutic Options and their Efficacy]". Fortschr Neurol Psychiatr (in German). 83 (4): 201–210. doi:10.1055/s-0034-1399338. PMID 25893493.
Addictive disorders are chronic relapsing conditions marked by compulsive and often uncontrolled use of psychotropic substances or stimuli. In this review, we present and discuss the current specific psychosocial interventions for addictive disorders and their effectiveness. In particular cognitive behavioral therapy, motivational interviewing, relapse prevention, the community reinforcement approach, and contingency management were found to be effective. For these psychotherapeutic treatments, mostly moderate effect sizes have been found. Their effectiveness seems to be highest in cannabis dependence. Empirical evidence for dependence on "hard" drugs is largest for contingency management, while for alcohol dependence motivational interviewing and the community reinforcement approach show the largest effect sizes. Presumably, combinations of different approaches as well as online interventions will bring further progress in the psychosocial treatment of addictive disorders in the future.
- ^ Zbukvic, Isabel C.; Ganella, Despina E.; Perry, Christina J.; Madsen, Heather B.; Bye, Christopher R.; Lawrence, Andrew J.; Kim, Jee Hyun (2016-03-05). "Role of Dopamine 2 Receptor in Impaired Drug-Cue Extinction in Adolescent Rats". Cerebral Cortex. 26: bhw051. doi:10.1093/cercor/bhw051. ISSN 1047-3211. PMC 4869820. PMID 26946126.
- ^ Carroll ME, Smethells JR (February 2016). "Sex Differences in Behavioral Dyscontrol: Role in Drug Addiction and Novel Treatments". Front. Psychiatry. 6: 175. doi:10.3389/fpsyt.2015.00175. PMC 4745113. PMID 26903885.
Environmental Enrichment ...
In humans, non-drug rewards delivered in a contingency management (CM) format successfully reduced drug dependence ... In general, CM programs promote drug abstinence through a combination of positive reinforcement for drug-free urine samples. For instance, voucher-based reinforcement therapy in which medication compliance, therapy session attendance, and negative drug screenings reinforced with vouchers to local business (e.g., movie theater, restaurants, etc.) directly reinforces drug abstinence, provides competing reinforcers, enriches the environment, and it is a robust treatment across a broad range of abused drugs (189). ...
Physical Exercise
There is accelerating evidence that physical exercise is a useful treatment for preventing and reducing drug addiction ... In some individuals, exercise has its own rewarding effects, and a behavioral economic interaction may occur, such that physical and social rewards of exercise can substitute for the rewarding effects of drug abuse. ... The value of this form of treatment for drug addiction in laboratory animals and humans is that exercise, if it can substitute for the rewarding effects of drugs, could be self-maintained over an extended period of time. Work to date in [laboratory animals and humans] regarding exercise as a treatment for drug addiction supports this hypothesis. ... However, a RTC study was recently reported by Rawson et al. (226), whereby they used 8 weeks of exercise as a post-residential treatment for METH addiction, showed a significant reduction in use (confirmed by urine screens) in participants who had been using meth 18 days or less a month. ... Animal and human research on physical exercise as a treatment for stimulant addiction indicates that this is one of the most promising treatments on the horizon. [emphasis added]{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA (September 2013). "Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis". Neurosci Biobehav Rev. 37 (8): 1622–44. doi:10.1016/j.neubiorev.2013.06.011. PMC 3788047. PMID 23806439.
[exercise] efficacy may be related to its ability to normalize glutamatergic and dopaminergic signaling and reverse drug-induced changes in chromatin via epigenetic interactions with brain-derived neurotrophic factor (BDNF) in the reward pathway. ... these data show that exercise can affect dopaminergic signaling at many different levels, which may underlie its ability to modify vulnerability during drug use initiation. Exercise also produces neuroadaptations that may influence an individual's vulnerability to initiate drug use. Consistent with this idea, chronic moderate levels of forced treadmill running blocks not only subsequent methamphetamine-induced conditioned place preference, but also stimulant-induced increases in dopamine release in the NAc (Chen et al., 2008) and striatum (Marques et al., 2008). ... [These] findings indicate the efficacy of exercise at reducing drug intake in drug-dependent individuals ... wheel running [reduces] methamphetamine self-administration under extended access conditions (Engelmann et al., 2013) ... These findings suggest that exercise may "magnitude"-dependently prevent the development of an addicted phenotype possibly by blocking/reversing behavioral and neuro-adaptive changes that develop during and following extended access to the drug. ... Exercise has been proposed as a treatment for drug addiction that may reduce drug craving and risk of relapse. Although few clinical studies have investigated the efficacy of exercise for preventing relapse, the few studies that have been conducted generally report a reduction in drug craving and better treatment outcomes (see Table 4). ... Taken together, these data suggest that the potential benefits of exercise during relapse, particularly for relapse to psychostimulants, may be mediated via chromatin remodeling and possibly lead to greater treatment outcomes.
- ^ a b Linke SE, Ussher M (2015). "Exercise-based treatments for substance use disorders: evidence, theory, and practicality". Am J Drug Alcohol Abuse. 41 (1): 7–15. doi:10.3109/00952990.2014.976708. PMID 25397661.
The limited research conducted suggests that exercise may be an effective adjunctive treatment for SUDs. In contrast to the scarce intervention trials to date, a relative abundance of literature on the theoretical and practical reasons supporting the investigation of this topic has been published. ... numerous theoretical and practical reasons support exercise-based treatments for SUDs, including psychological, behavioral, neurobiological, nearly universal safety profile, and overall positive health effects.
- ^ a b Zhou Y, Zhao M, Zhou C, Li R (July 2015). "Sex differences in drug addiction and response to exercise intervention: From human to animal studies". Front. Neuroendocrinol. 40: 24–41. doi:10.1016/j.yfrne.2015.07.001. PMID 26182835.
Collectively, these findings demonstrate that exercise may serve as a substitute or competition for drug abuse by changing ΔFosB or cFos immunoreactivity in the reward system to protect against later or previous drug use. ... As briefly reviewed above, a large number of human and rodent studies clearly show that there are sex differences in drug addiction and exercise. The sex differences are also found in the effectiveness of exercise on drug addiction prevention and treatment, as well as underlying neurobiological mechanisms. The postulate that exercise serves as an ideal intervention for drug addiction has been widely recognized and used in human and animal rehabilitation. ... In particular, more studies on the neurobiological mechanism of exercise and its roles in preventing and treating drug addiction are needed.
- ^ Soyka M, Roesner S (2006). "New pharmacological approaches for the treatment of alcoholism". Expert Opin Pharmacother. 7 (17): 2341–53. doi:10.1517/14656566.7.17.2341. PMID 17109610.
- ^ Pettinati HM, Rabinowitz AR (2006). "Choosing the right medication for the treatment of alcoholism". Curr Psychiatry Rep. 8 (5): 383–8. doi:10.1007/s11920-006-0040-0. PMID 16968619.
- ^ Bouza C, Angeles M, Magro A, Muñoz A, Amate JM (2004). "Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review". Addiction. 99 (7): 811–28. doi:10.1111/j.1360-0443.2004.00763.x. PMID 15200577.
- ^ Williams SH (2005). "Medications for treating alcohol dependence". Am Fam Physician. 72 (9): 1775–80. PMID 16300039.
- ^ Srisurapanont M; Jarusuraisin N (2005). Srisurapanont, Manit (ed.). "Opioid antagonists for alcohol dependence". Cochrane Database Syst Rev (1): CD001867. doi:10.1002/14651858.CD001867.pub2. PMID 15674887.
- ^ Smith, David E. (2012-01-01). "Editor's Note: The Process Addictions and the New ASAM Definition of Addiction". Journal of Psychoactive Drugs. 44 (1): 1–4. doi:10.1080/02791072.2012.662105. ISSN 0279-1072. PMID 22641960.
- ^ American Psychiatric Association (2022-03-18). Diagnostic and Statistical Manual of Mental Disorders (DSM-5-TR ed.). American Psychiatric Association Publishing. p. 661. doi:10.1176/appi.books.9780890425787. ISBN 978-0-89042-575-6. S2CID 249488050.
- ^ Stein, Dan J.; Hollander, Eric; Rothbaum, Barbara Olasov (31 August 2009). Textbook of Anxiety Disorders. American Psychiatric Pub. pp. 359–. ISBN 978-1-58562-254-2. Retrieved 24 April 2010.
- ^ Holden, Constance (2001-11-02). "'Behavioral' Addictions: Do They Exist?". Science. 294 (5544): 980–982. doi:10.1126/science.294.5544.980. ISSN 0036-8075. PMID 11691967. S2CID 27235598.
- ^ Starcevic, Vladan (August 2016). "Behavioural addictions: A challenge for psychopathology and psychiatric nosology". The Australian and New Zealand Journal of Psychiatry. 50 (8): 721–725. doi:10.1177/0004867416654009. ISSN 1440-1614. PMID 27357713. S2CID 22843151.
- ^ Pinna, F.; Dell’Osso, B.; Di Nicola, M.; Janiri, L.; Altamura, A.C.; Carpiniello, B.; Hollander, E. (1 Dec 2015). "Behavioural addictions and the transition from DSM-IV-TR to DSM-5" (PDF). Journal of Psychopathology. 21 (4): 380–389.
- ^ Petry, Nancy (2015). Behavioral Addictions: DSM-5 and Beyond. Oxford University Press. pp. 1–5. ISBN 9780199391554.
- ^ Kuss, Daria (2013). "Internet gaming addiction: current perspectives". Psychology Research and Behavior Management. 6 (6): 125–137. doi:10.2147/PRBM.S39476. PMC 3832462. PMID 24255603.
- ^ American Psychiatric Association (2022-03-18). Diagnostic and Statistical Manual of Mental Disorders (DSM-5-TR ed.). American Psychiatric Association Publishing. p. 914. doi:10.1176/appi.books.9780890425787. ISBN 978-0-89042-575-6. S2CID 249488050.
- ^ a b American Psychiatric Association (2022-03-18). Diagnostic and Statistical Manual of Mental Disorders (DSM-5-TR ed.). American Psychiatric Association Publishing. p. 543. doi:10.1176/appi.books.9780890425787. ISBN 978-0-89042-575-6. S2CID 249488050.
- ^ a b Stein, Dan J.; Szatmari, Peter; Gaebel, Wolfgang; Berk, Michael; Vieta, Eduard; Maj, Mario; de Vries, Ymkje Anna; Roest, Annelieke M.; de Jonge, Peter; Maercker, Andreas; Brewin, Chris R.; Pike, Kathleen M.; Grilo, Carlos M.; Fineberg, Naomi A.; Briken, Peer (27 January 2020). "Mental, behavioral and neurodevelopmental disorders in the ICD-11: an international perspective on key changes and controversies". BMC Medicine. 18 (1): 21. doi:10.1186/s12916-020-1495-2. ISSN 1741-7015. PMC 6983973. PMID 31983345.
- ^ "International Classification of Diseases Eleventh Revision (ICD-11)". World Health Organization. Geneva. 2022. Retrieved 2023-10-05.
- ^ "What is the Definition of Addiction?" (PDF). American Society of Addiction Medicine. 15 September 2019. Archived from the original on 15 September 2019. Retrieved 2023-10-05.
- ^ a b Brand, Matthias; Rumpf, Hans-JÜrgen; Demetrovics, Zsolt; MÜller, Astrid; Stark, Rudolf; King, Daniel L.; Goudriaan, Anna E.; Mann, Karl; Trotzke, Patrick; Fineberg, Naomi A.; Chamberlain, Samuel R.; Kraus, Shane W.; Wegmann, Elisa; Billieux, JoËl; Potenza, Marc N. (2020-06-30). "Which conditions should be considered as disorders in the International Classification of Diseases (ICD-11) designation of "other specified disorders due to addictive behaviors"?". Journal of Behavioral Addictions. 11 (2): 150–159. doi:10.1556/2006.2020.00035. ISSN 2062-5871. PMC 9295220. PMID 32634114.
- ^ a b Griffiths, Mark D. (2022-07-13). "Disorders due to addictive behaviors: Further issues, debates, and controversies •: Commentary to the debate: "Behavioral addictions in the ICD-11"". Journal of Behavioral Addictions. 11 (2): 180–185. doi:10.1556/2006.2022.00025. ISSN 2062-5871. PMC 9295243. PMID 35895451.
- ^ Hauck, Carolin; Cook, Brian; Ellrott, Thomas (20 November 2019). "Food addiction, eating addiction and eating disorders". Proceedings of the Nutrition Society. 79 (1). Cambridge University Press: 103–112. doi:10.1017/S0029665119001162. ISSN 0029-6651. PMID 31744566. S2CID 208186539.
- ^ Rosenberg, Kenneth Paul; Feder, Laura Curtiss (2014), "An Introduction to Behavioral Addictions", Behavioral Addictions, Elsevier, pp. 1–17, doi:10.1016/b978-0-12-407724-9.00001-x, ISBN 9780124077249, retrieved 2023-11-03
- ^ Bannink, F. P. (2013-06-26). "Positive CBT: From Reducing Distress to Building Success". Journal of Contemporary Psychotherapy. 44 (1): 1–8. doi:10.1007/s10879-013-9239-7. ISSN 0022-0116. S2CID 24029889.
- ^ Better Life Recovery
- ^ Litchke, Lyn Gorbett; Quinn, Bonnie; Turner, Kassandra; Trapp, Kelci (2022-04-03). "Therapeutic Recreation Activities Combined with a 12-Step Faith-Based Program for Adults Experiencing Addiction, Mental Health, and Homelessness: A Case Study". Alcoholism Treatment Quarterly. 40 (2): 217–228. doi:10.1080/07347324.2021.1981181. ISSN 0734-7324.
- ^ Staff, Lakeview Health (2021-07-19). "How Therapeutic Recreation Helps With Addiction Treatment". Lakeview Health. Retrieved 2024-10-22.
- ^ Billieux, Joël; Schimmenti, Adriano; Khazaal, Yasser; Maurage, Pierre; Heeren, Alexandre (2015-05-27). "Are we overpathologizing everyday life? A tenable blueprint for behavioral addiction research". Journal of Behavioral Addictions. 4 (3): 119–123. doi:10.1556/2006.4.2015.009. ISSN 2062-5871. PMC 4627665. PMID 26014667.
- ^ Starcevic, Vladan; Khazaal, Yasser (2017-04-07). "Relationships between Behavioural Addictions and Psychiatric Disorders: What Is Known and What Is Yet to Be Learned?". Frontiers in Psychiatry. 8: 53. doi:10.3389/fpsyt.2017.00053. ISSN 1664-0640. PMC 5383701. PMID 28439243.
- ^ Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM (February 2013). "Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator". J. Neurosci. 33 (8): 3434–42. doi:10.1523/JNEUROSCI.4881-12.2013. PMC 3865508. PMID 23426671.
Together, these findings demonstrate that drugs of abuse and natural reward behaviors act on common molecular and cellular mechanisms of plasticity that control vulnerability to drug addiction, and that this increased vulnerability is mediated by ΔFosB and its downstream transcriptional targets.
- ^ Brewer, Judson A.; Potenza, Marc N. (2008). "The neurobiology and genetics of impulse control disorders: Relationships to drug addictions". Biochemical Pharmacology. 75 (1): 63–75. doi:10.1016/j.bcp.2007.06.043. PMC 2222549. PMID 17719013.
- ^ Girault, Jean-Antoine; Greengard, P (2004). "The Neurobiology of Dopamine Signaling". Archives of Neurology. 61 (5): 641–4. doi:10.1001/archneur.61.5.641. PMID 15148138.
- ^ a b Dichiara, G; Bassareo, V (2007). "Reward system and addiction: What dopamine does and doesn't do". Current Opinion in Pharmacology. 7 (1): 69–76. doi:10.1016/j.coph.2006.11.003. PMID 17174602.
External links
 Media related to Addictions at Wikimedia Commons
Media related to Addictions at Wikimedia Commons- Valerie Voon – Impulse control disorders – behavioural addictions – insights from dopaminergic ... on YouTube Technical review of biomolecular-neurobehavioral research
Cannabinoid addiction
As of 2010[update], there are no effective pharmacological interventions for cannabinoid addiction.[1] A 2013 review on cannabinoid addiction noted that the development of CB1 receptor agonists that have reduced interaction with β-arrestin 2 signaling might be therapeutically useful.[2]
Nicotine addiction
Another area in which drug treatment has been widely used is in the treatment of nicotine addiction, which usually involves the use of nicotine replacement therapy, nicotinic receptor antagonists, or nicotinic receptor partial agonists.[3][4] Examples of drugs that act on nicotinic receptors and have been used for treating nicotine addiction include antagonists like bupropion and the partial agonist varenicline.[3][4]
Opioid addiction
This section needs more reliable medical references for verification or relies too heavily on primary sources. (April 2016) |  |
Opioids cause physical dependence, and treatment typically addresses both dependence and addiction.
Physical dependence is treated using replacement drugs such as suboxone or subutex (both containing the active ingredients buprenorphine) and methadone).[5][6] Although these drugs perpetuate physical dependence, the goal of opiate maintenance is to provide a measure of control over both pain and cravings. Use of replacement drugs increases the patient's ability to function normally and eliminates the negative consequences of obtaining controlled substances illicitly. Once a prescribed dosage is stabilized, treatment enters maintenance or tapering phases. In the United States, opiate replacement therapy is tightly regulated in methadone clinics and under the DATA 2000 legislation. In some countries, other opioid derivatives such as levomethadyl acetate,[7] dihydrocodeine,[8] dihydroetorphine[9] and even heroin[10][11] are used as substitute drugs for illegal street opiates, with different prescriptions being given depending on the needs of the individual patient. Baclofen has led to successful reductions of cravings for stimulants, alcohol, and opioids, and also alleviates alcohol withdrawal syndrome. Many patients have stated they "became indifferent to alcohol" or "indifferent to cocaine" overnight after starting baclofen therapy.[12]
Psychostimulant addiction
As of May 2014[update], there is no effective pharmacotherapy for any form of psychostimulant addiction.[13][14][15][16] Reviews from 2015 and 2016 indicated that TAAR1-selective agonists have significant therapeutic potential as a treatment for psychostimulant addictions;[17][18] however, as of February 2016[update], the only compounds which are known to function as TAAR1-selective agonists are experimental drugs.[17][18]
Research
This section needs expansion. You can help by adding to it. (April 2016) |
Research indicates that vaccines which utilize anti-drug monoclonal antibodies can mitigate drug-induced positive reinforcement by preventing the drug from moving across the blood–brain barrier;[19] however, current vaccine-based therapies are only effective in a relatively small subset of individuals.[19][20] As of November 2015[update], vaccine-based therapies are being tested in human clinical trials as a treatment for addiction and preventative measure against drug overdoses involving nicotine, cocaine, and methamphetamine.[19]
Since addiction involves abnormalities in glutamate and GABAergic neurotransmission,[21][22] receptors associated with these neurotransmitters (e.g., AMPA receptors, NMDA receptors, and GABAB receptors) are potential therapeutic targets for addictions.[21][22][23][24] N-acetylcysteine, which affects metabotropic glutamate receptors and NMDA receptors, has shown some benefit in preclinical and clinical studies involving addictions to cocaine, heroin, and cannabinoids.[21] It may also be useful as an adjunct therapy for addictions to amphetamine-type stimulants, but more clinical research is required.[21]
Current medical reviews of research involving lab animals have identified a drug class – class I histone deacetylase inhibitors[note 6] – that indirectly inhibits the function and further increases in the expression of accumbal ΔFosB by inducing G9a expression in the nucleus accumbens after prolonged use.[27][25][26][28] These reviews and subsequent preliminary evidence which used oral administration or intraperitoneal administration of the sodium salt of butyric acid or other class I HDAC inhibitors for an extended period indicate that these drugs have efficacy in reducing addictive behavior in lab animals[note 7] that have developed addictions to ethanol, psychostimulants (i.e., amphetamine and cocaine), nicotine, and opiates;[26][28][30][29] however, as of August 2015[update] no clinical trials involving human addicts and any HDAC class I inhibitors have been conducted to test for treatment efficacy in humans or identify an optimal dosing regimen.
Epidemiology
Due to cultural variations, the proportion of individuals who develop a drug or behavioral addiction within a specified time period (i.e., the prevalence) varies over time, by country, and across national population demographics (e.g., by age group, socioeconomic status, etc.).[31]
Asia
This section is empty. You can help by adding to it. (December 2015) |
Australia
This section is empty. You can help by adding to it. (December 2015) |
Europe
This section is empty. You can help by adding to it. (December 2015) |
United States
This section is missing information about prescription drug addiction prevalence rate(s). (June 2015) |
Based upon representative samples of the US youth population in 2011, the lifetime prevalence[note 8] of addictions to alcohol and illicit drugs has been estimated to be approximately 8% and 2–3% respectively.[32] Based upon representative samples of US adult population in 2011, the 12 month prevalence of alcohol and illicit drug addictions were estimated at roughly 12% and 2–3% respectively.[32] The 12 month and lifetime prevalence of prescription drug addictions is currently unknown.
As of 2016[update], about 22 million Americans need treatment for an addiction to drugs.[33][34] Only about 10%, or a little over 2 million, receive any form of treatments, and those that do generally do not receive evidence-based care.[33][34] One-third of inpatient hospital costs and 20% of all deaths in the US every year are the result of untreated addictions and risky substance use.[33][34] In spite of the massive overall economic cost to society, which is greater than the cost of diabetes and all forms of cancer combined, most doctors in the US lack the training to effectively address a drug addiction.[33][34]
Another review listed estimates of lifetime prevalence rates for several behavioral addictions in the United States, including 1–2% for compulsive gambling, 5% for sexual addiction, 2.8% for food addiction, and 5–6% for compulsive shopping.[35] A systematic review indicated that the time-invariant prevalence rate for sexual addiction and related compulsive sexual behavior (e.g., compulsive masturbation with or without pornography, compulsive cybersex, etc.) within the United States ranges from 3–6% of the population.[36]
Personality theories of addiction
Personality theories of addiction are psychological models that associate personality traits or modes of thinking (i.e., affective states) with an individual's proclivity for developing an addiction. Models of addiction risk that have been proposed in psychology literature include an affect dysregulation model of positive and negative psychological affects, the reinforcement sensitivity theory model of impulsiveness and behavioral inhibition, and an impulsivity model of reward sensitization and impulsiveness.[37][38][39][40][41]
Notes
- ^ a b A decrease in aversion sensitivity, in simpler terms, means that an individual's behavior is less likely to be influenced by undesirable outcomes.
- ^ In other words, c-Fos repression allows ΔFosB to accumulate within nucleus accumbens medium spiny neurons more rapidly because it is selectively induced in this state.[1]
- ^ According to two medical reviews, ΔFosB has been implicated in causing both increases and decreases in dynorphin expression in different studies;[10][69] this table entry reflects only a decrease.
- ^ Incentive salience, the "motivational salience" for a reward, is a "desire" or "want" attribute, which includes a motivational component, that the brain assigns to a rewarding stimulus.[70][71] As a consequence, incentive salience acts as a motivational "magnet" for a rewarding stimulus that commands attention, induces approach, and causes the rewarding stimulus to be sought out.[70]
- ^ In simplest terms, this means that when either amphetamine or sex is perceived as more alluring or desirable through reward sensitization, this effect occurs with the other as well.
- ^ Inhibitors of class I histone deacetylase (HDAC) enzymes are drugs that inhibit four specific histone-modifying enzymes: HDAC1, HDAC2, HDAC3, and HDAC8. Most of the animal research with HDAC inhibitors has been conducted with four drugs: butyrate salts (mainly sodium butyrate), trichostatin A, valproic acid, and SAHA;[25][26] butyric acid is a naturally occurring short-chain fatty acid in humans, while the latter two compounds are FDA-approved drugs with medical indications unrelated to addiction.
- ^ Specifically, prolonged administration of a class I HDAC inhibitor appears to reduce an animal's motivation to acquire and use an addictive drug without affecting an animals motivation to attain other rewards (i.e., it does not appear to cause motivational anhedonia) and reduce the amount of the drug that is self-administered when it is readily available.[26][28][29]
- ^ The lifetime prevalence of an addiction is the percentage of individuals in a population (the one which the sample represents) that developed an addiction at some point in their life, at time of assessment.
Image legend
- ^ (Text color) Transcription factors
References
- ^ Sofuoglu M, Sugarman DE, Carroll KM (April 2010). "Cognitive function as an emerging treatment target for marijuana addiction". Exp Clin Psychopharmacol. 18 (2): 109–119. doi:10.1037/a0019295. PMC 2909584. PMID 20384422.
Cannabis is the most widely used illicit substance in the world, and demand for effective treatment is increasing. However, abstinence rates following behavioral therapies have been modest, and there are no effective pharmacotherapies for the treatment of cannabis addiction.
- ^ Fratta W, Fattore L (August 2013). "Molecular mechanisms of cannabinoid addiction". Curr. Opin. Neurobiol. 23 (4): 487–492. doi:10.1016/j.conb.2013.02.002. PMID 23490548.
14. Nguyen PT, Schmid CL, Raehal KM, Selley DE, Bohn LM, Sim-Selley LJ: b-Arrestin2 regulates cannabinoid CB1 receptor signaling and adaptation in a central nervous system region dependent manner. Biol Psychiatry 2012, 71:714-724.
A pioneering study revealing both positive and negative modulatory effects of beta-arrestin2 on THC tolerance. By demonstrating that tolerance to antinociception is reduced whereas tolerance to catalepsy is enhanced in beta-arrestin2 knockout mice, authors suggest that development of cannabinoid agonists that minimize interactions between CB1Rs and beta-arrestin2 might produce improved cannabinoid analgesics with reduced motor suppression, and be therapeutically beneficial. - ^ a b Garwood CL, Potts LA (2007). "Emerging pharmacotherapies for smoking cessation". Am J Health Syst Pharm. 64 (16): 1693–8. doi:10.2146/ajhp060427. PMID 17687057.
- ^ a b Crooks PA, Bardo MT, Dwoskin LP (2014). "Nicotinic receptor antagonists as treatments for nicotine abuse". Adv. Pharmacol. 69: 513–51. doi:10.1016/B978-0-12-420118-7.00013-5. PMC 4110698. PMID 24484986.
- ^ Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE (2000). "A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence". N. Engl. J. Med. 343 (18): 1290–7. doi:10.1056/NEJM200011023431802. PMID 11058673.
- ^ Connock M, Juarez-Garcia A, Jowett S, et al. (2007). "Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation". Health Technol Assess. 11 (9): 1–171, iii–iv. doi:10.3310/hta11090. PMID 17313907.
- ^ Marsch LA, Stephens MA, Mudric T, Strain EC, Bigelow GE, Johnson RE (2005). "Predictors of outcome in LAAM, buprenorphine, and methadone treatment for opioid dependence". Exp Clin Psychopharmacol. 13 (4): 293–302. doi:10.1037/1064-1297.13.4.293. PMID 16366759.
- ^ Robertson JR, Raab GM, Bruce M, McKenzie JS, Storkey HR, Salter A (2006). "Addressing the efficacy of dihydrocodeine versus methadone as an alternative maintenance treatment for opiate dependence: A randomized controlled trial". Addiction. 101 (12): 1752–9. doi:10.1111/j.1360-0443.2006.01603.x. PMID 17156174.
- ^ Qin Bo-Yi (1998). "Advances in dihydroetorphine: From analgesia to detoxification". Drug Development Research. 39 (2): 131–134. doi:10.1002/(SICI)1098-2299(199610)39:2<131::AID-DDR3>3.0.CO;2-Q. Link
- ^ Metrebian N, Shanahan W, Wells B, Stimson GV (1998). "Feasibility of prescribing injectable heroin and methadone to opiate-dependent drug users: associated health gains and harm reductions". Med. J. Aust. 168 (12): 596–600. PMID 9673620.
- ^ Metrebian N, Mott J, Carnwath Z, Carnwath T, Stimson GV, Sell L (2007). "Pathways into receiving a prescription for diamorphine (heroin) for the treatment of opiate dependence in the United kingdom". Eur Addict Res. 13 (3): 144–7. doi:10.1159/000101550. PMID 17570910.
- ^ Kenna GA, Nielsen DM, Mello P, Schiesl A, Swift RM (2007). "Pharmacotherapy of dual substance abuse and dependence". CNS Drugs. 21 (3): 213–37. doi:10.2165/00023210-200721030-00003. PMID 17338593.
- ^ "The neurocircuitry of illicit psychostimulant addiction: acute and chronic effects in humans". Subst. Abuse Rehabil. 4: 29–43. February 2013. doi:10.2147/SAR.S39684. PMC 3931688. PMID 24648786.
Initial drug use can be attributed to the ability of the drug to act as a reward (ie, a pleasurable emotional state or positive reinforcer), which can lead to repeated drug use and dependence.8,9 A great deal of research has focused on the molecular and neuroanatomical mechanisms of the initial rewarding or reinforcing effect of drugs of abuse. ... At present, no pharmacological therapy has been approved by the FDA to treat psychostimulant addiction. Many drugs have been tested, but none have shown conclusive efficacy with tolerable side effects in humans.172 These drugs have included DA-receptor ligands, such as DA receptor agonists, partial agonists, and antagonists, as well as DA-reuptake inhibitors.173,174 One newer dopaminergic drug that has shown some promise is the antipsychotic medication aripiprazole, a partial agonist at D2-like receptors, which is currently approved for the treatment of schizophrenia, depression, and bipolar disorder.175 Clinical trials have thus far been mixed, with one study finding reduced cocaine craving and use, and another study showing increased cocaine use.176,177 ... In addition to the pharmacological trials reviewed above, less conventional strategies that are gaining scientific momentum include vaccine therapies to immunoneutralize drug molecules and impede penetrance across the blood–brain barrier, enzyme conjugates that dramatically increase the metabolic breakdown of abused drugs, pharmacogenetic approaches based on individual genetic polymorphisms in addiction-related genes, and epigenetic modulators of drug-induced changes in gene expression.208–211 While still in their relative infancy, these exciting new avenues of research offer a significant expansion of possible biologically based targets for the treatment of psychostimulant addiction. ... The tremendous need for more effective pharmacological treatments for psychostimulant addiction is a mainstay of contemporary addiction research. However, the recent downsizing of many major pharmaceutical companies away from psychiatric indications (including addiction) due to the lack of efficacy of experimental compounds in humans may require a sea change in the translational research approach.212,213 A new emphasis on larger-scale biomarker, genetic, and epigenetic research focused on the molecular targets of mental disorders has been recently advocated.212 In addition, the integration of cognitive and behavioral modification of circuit-wide neuroplasticity (ie, computer-based training to enhance executive function) may prove to be an effective adjunct-treatment approach for addiction, particularly when combined with cognitive enhancers.198,213–216 Furthermore, in order to be effective, all pharmacological or biologically based treatments for addiction need to be integrated into other established forms of addiction rehabilitation, such as cognitive behavioral therapy, individual and group psychotherapy, behavior-modification strategies, twelve-step programs, and residential treatment facilities.
{{cite journal}}: Unknown parameter|authors=ignored (help)CS1 maint: unflagged free DOI (link) - ^ Stoops WW, Rush CR (May 2014). "Combination pharmacotherapies for stimulant use disorder: a review of clinical findings and recommendations for future research". Expert Rev Clin Pharmacol. 7 (3): 363–374. doi:10.1586/17512433.2014.909283. PMID 24716825.
Despite concerted efforts to identify a pharmacotherapy for managing stimulant use disorders, no widely effective medications have been approved.
- ^ Perez-Mana C, Castells X, Torrens M, Capella D, Farre M (September 2013). "Efficacy of psychostimulant drugs for amphetamine abuse or dependence". Cochrane Database Syst. Rev. 9: CD009695. doi:10.1002/14651858.CD009695.pub2. PMID 23996457.
To date, no pharmacological treatment has been approved for [addiction], and psychotherapy remains the mainstay of treatment. ... Results of this review do not support the use of psychostimulant medications at the tested doses as a replacement therapy
- ^ Forray A, Sofuoglu M (February 2014). "Future pharmacological treatments for substance use disorders". Br. J. Clin. Pharmacol. 77 (2): 382–400. doi:10.1111/j.1365-2125.2012.04474.x. PMC 4014020. PMID 23039267.
- ^ a b Grandy DK, Miller GM, Li JX (February 2016). ""TAARgeting Addiction"-The Alamo Bears Witness to Another Revolution: An Overview of the Plenary Symposium of the 2015 Behavior, Biology and Chemistry Conference". Drug Alcohol Depend. 159: 9–16. doi:10.1016/j.drugalcdep.2015.11.014. PMID 26644139.
When considered together with the rapidly growing literature in the field a compelling case emerges in support of developing TAAR1-selective agonists as medications for preventing relapse to psychostimulant abuse.
- ^ a b Jing L, Li JX (August 2015). "Trace amine-associated receptor 1: A promising target for the treatment of psychostimulant addiction". Eur. J. Pharmacol. 761: 345–352. doi:10.1016/j.ejphar.2015.06.019. PMID 26092759.
Taken together,the data reviewed here strongly support that TAAR1 is implicated in the functional regulation of monoaminergic systems, especially dopaminergic system, and that TAAR1 serves as a homeostatic "brake" system that is involved in the modulation of dopaminergic activity. Existing data provided robust preclinical evidence supporting the development of TAAR1 agonists as potential treatment for psychostimulant abuse and addiction. ... Given that TAAR1 is primarily located in the intracellular compartments and existing TAAR1 agonists are proposed to get access to the receptors by translocation to the cell interior (Miller, 2011), future drug design and development efforts may need to take strategies of drug delivery into consideration (Rajendran et al., 2010).
- ^ a b c Zalewska-Kaszubska J (November 2015). "Is immunotherapy an opportunity for effective treatment of drug addiction?". Vaccine. 33 (48): 6545–6551. doi:10.1016/j.vaccine.2015.09.079. PMID 26432911.
- ^ Laudenbach M, Baruffaldi F, Vervacke JS, Distefano MD, Titcombe PJ, Mueller DL, Tubo NJ, Griffith TS, Pravetoni M (June 2015). "The frequency of naive and early-activated hapten-specific B cell subsets dictates the efficacy of a therapeutic vaccine against prescription opioid abuse". J. Immunol. 194 (12): 5926–5936. doi:10.4049/jimmunol.1500385. PMID 25972483.
Translation of therapeutic vaccines for addiction, cancer, or other chronic noncommunicable diseases has been slow because only a small subset of immunized subjects achieved effective Ab levels.
- ^ a b c d Cao DN, Shi JJ, Hao W, Wu N, Li J (March 2016). "Advances and challenges in pharmacotherapeutics for amphetamine-type stimulants addiction". Eur. J. Pharmacol. 780: 129–35. doi:10.1016/j.ejphar.2016.03.040. PMID 27018393.
- ^ a b Moeller SJ, London ED, Northoff G (February 2016). "Neuroimaging markers of glutamatergic and GABAergic systems in drug addiction: Relationships to resting-state functional connectivity". Neurosci Biobehav Rev. 61: 35–52. doi:10.1016/j.neubiorev.2015.11.010. PMID 26657968.
- ^ Agabio R, Colombo G (April 2015). "[GABAB receptor as therapeutic target for drug addiction: from baclofen to positive allosteric modulators]". Psychiatr. Pol. (in Polish). 49 (2): 215–223. doi:10.12740/PP/33911. PMID 26093587.
- ^ Filip M, Frankowska M, Sadakierska-Chudy A, Suder A, Szumiec L, Mierzejewski P, Bienkowski P, Przegaliński E, Cryan JF (January 2015). "GABAB receptors as a therapeutic strategy in substance use disorders: focus on positive allosteric modulators". Neuropharmacology. 88: 36–47. doi:10.1016/j.neuropharm.2014.06.016. PMID 24971600.
- ^ a b McCowan TJ, Dhasarathy A, Carvelli L (February 2015). "The Epigenetic Mechanisms of Amphetamine". J. Addict. Prev. (S1). Avens Publishing Group: 1–7. ISSN 2330-2178. Retrieved 30 April 2015.
Epigenetic modifications caused by addictive drugs play an important role in neuronal plasticity and in drug-induced behavioral responses. Although few studies have investigated the effects of AMPH on gene regulation (Table 1), current data suggest that AMPH acts at multiple levels to alter histone/DNA interaction and to recruit transcription factors which ultimately cause repression of some genes and activation of other genes. Importantly, some studies have also correlated the epigenetic regulation induced by AMPH with the behavioral outcomes caused by this drug, suggesting therefore that epigenetics remodeling underlies the behavioral changes induced by AMPH. If this proves to be true, the use of specific drugs that inhibit histone acetylation, methylation or DNA methylation might be an important therapeutic alternative to prevent and/or reverse AMPH addiction and mitigate the side effects generate by AMPH when used to treat ADHD.
- ^ a b c d Walker DM, Cates HM, Heller EA, Nestler EJ (February 2015). "Regulation of chromatin states by drugs of abuse". Curr. Opin. Neurobiol. 30: 112–121. doi:10.1016/j.conb.2014.11.002. PMID 25486626.
Studies investigating general HDAC inhibition on behavioral outcomes have produced varying results but it seems that the effects are specific to the timing of exposure (either before, during or after exposure to drugs of abuse) as well as the length of exposure
- ^ Cite error: The named reference
G9a reverses ΔFosB plasticitywas invoked but never defined (see the help page). - ^ a b c Nestler EJ (January 2014). "Epigenetic mechanisms of drug addiction". Neuropharmacology. 76 Pt B: 259–268. doi:10.1016/j.neuropharm.2013.04.004. PMC 3766384. PMID 23643695.
Short-term increases in histone acetylation generally promote behavioral responses to the drugs, while sustained increases oppose cocaine's effects, based on the actions of systemic or intra-NAc administration of HDAC inhibitors. ... Genetic or pharmacological blockade of G9a in the NAc potentiates behavioral responses to cocaine and opiates, whereas increasing G9a function exerts the opposite effect (Maze et al., 2010; Sun et al., 2012a). Such drug-induced downregulation of G9a and H3K9me2 also sensitizes animals to the deleterious effects of subsequent chronic stress (Covington et al., 2011). Downregulation of G9a increases the dendritic arborization of NAc neurons, and is associated with increased expression of numerous proteins implicated in synaptic function, which directly connects altered G9a/H3K9me2 in the synaptic plasticity associated with addiction (Maze et al., 2010).
G9a appears to be a critical control point for epigenetic regulation in NAc, as we know it functions in two negative feedback loops. It opposes the induction of ΔFosB, a long-lasting transcription factor important for drug addiction (Robison and Nestler, 2011), while ΔFosB in turn suppresses G9a expression (Maze et al., 2010; Sun et al., 2012a). ... Also, G9a is induced in NAc upon prolonged HDAC inhibition, which explains the paradoxical attenuation of cocaine's behavioral effects seen under these conditions, as noted above (Kennedy et al., 2013). GABAA receptor subunit genes are among those that are controlled by this feedback loop. Thus, chronic cocaine, or prolonged HDAC inhibition, induces several GABAA receptor subunits in NAc, which is associated with increased frequency of inhibitory postsynaptic currents (IPSCs). In striking contrast, combined exposure to cocaine and HDAC inhibition, which triggers the induction of G9a and increased global levels of H3K9me2, leads to blockade of GABAA receptor and IPSC regulation. - ^ a b Primary references involving sodium butyrate:
• Kennedy PJ, Feng J, Robison AJ, Maze I, Badimon A, Mouzon E, Chaudhury D, Damez-Werno DM, Haggarty SJ, Han MH, Bassel-Duby R, Olson EN, Nestler EJ (April 2013). "Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation". Nat. Neurosci. 16 (4): 434–440. doi:10.1038/nn.3354. PMC 3609040. PMID 23475113.While acute HDAC inhibition enhances the behavioral effects of cocaine or amphetamine1,3,4,13,14, studies suggest that more chronic regimens block psychostimulant-induced plasticity3,5,11,12. ... The effects of pharmacological inhibition of HDACs on psychostimulant-induced plasticity appear to depend on the timecourse of HDAC inhibition. Studies employing co-administration procedures in which inhibitors are given acutely, just prior to psychostimulant administration, report heightened behavioral responses to the drug1,3,4,13,14. In contrast, experimental paradigms like the one employed here, in which HDAC inhibitors are administered more chronically, for several days prior to psychostimulant exposure, show inhibited expression3 or decreased acquisition of behavioral adaptations to drug5,11,12. The clustering of seemingly discrepant results based on experimental methodologies is interesting in light of our present findings. Both HDAC inhibitors and psychostimulants increase global levels of histone acetylation in NAc. Thus, when co-administered acutely, these drugs may have synergistic effects, leading to heightened transcriptional activation of psychostimulant-regulated target genes. In contrast, when a psychostimulant is given in the context of prolonged, HDAC inhibitor-induced hyperacetylation, homeostatic processes may direct AcH3 binding to the promoters of genes (e.g., G9a) responsible for inducing chromatin condensation and gene repression (e.g., via H3K9me2) in order to dampen already heightened transcriptional activation. Our present findings thus demonstrate clear cross talk among histone PTMs and suggest that decreased behavioral sensitivity to psychostimulants following prolonged HDAC inhibition might be mediated through decreased activity of HDAC1 at H3K9 KMT promoters and subsequent increases in H3K9me2 and gene repression.
• Simon-O'Brien E, Alaux-Cantin S, Warnault V, Buttolo R, Naassila M, Vilpoux C (July 2015). "The histone deacetylase inhibitor sodium butyrate decreases excessive ethanol intake in dependent animals". Addict Biol. 20 (4): 676–689. doi:10.1111/adb.12161. PMID 25041570.Altogether, our results clearly demonstrated the efficacy of NaB in preventing excessive ethanol intake and relapse and support the hypothesis that HDACi may have a potential use in alcohol addiction treatment.
• Castino MR, Cornish JL, Clemens KJ (April 2015). "Inhibition of histone deacetylases facilitates extinction and attenuates reinstatement of nicotine self-administration in rats". PLoS ONE. 10 (4): e0124796. doi:10.1371/journal.pone.0124796. PMC 4399837. PMID 25880762.treatment with NaB significantly attenuated nicotine and nicotine + cue reinstatement when administered immediately ... These results provide the first demonstration that HDAC inhibition facilitates the extinction of responding for an intravenously self-administered drug of abuse and further highlight the potential of HDAC inhibitors in the treatment of drug addiction.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Kyzar EJ, Pandey SC (August 2015). "Molecular mechanisms of synaptic remodeling in alcoholism". Neurosci. Lett. 601: 11–9. doi:10.1016/j.neulet.2015.01.051. PMID 25623036.
Increased HDAC2 expression decreases the expression of genes important for the maintenance of dendritic spine density such as BDNF, Arc, and NPY, leading to increased anxiety and alcohol-seeking behavior. Decreasing HDAC2 reverses both the molecular and behavioral consequences of alcohol addiction, thus implicating this enzyme as a potential treatment target (Fig. 3). HDAC2 is also crucial for the induction and maintenance of structural synaptic plasticity in other neurological domains such as memory formation [115]. Taken together, these findings underscore the potential usefulness of HDAC inhibition in treating alcohol use disorders ... Given the ability of HDAC inhibitors to potently modulate the synaptic plasticity of learning and memory [118], these drugs hold potential as treatment for substance abuse-related disorders. ... Our lab and others have published extensively on the ability of HDAC inhibitors to reverse the gene expression deficits caused by multiple models of alcoholism and alcohol abuse, the results of which were discussed above [25,112,113]. This data supports further examination of histone modifying agents as potential therapeutic drugs in the treatment of alcohol addiction ... Future studies should continue to elucidate the specific epigenetic mechanisms underlying compulsive alcohol use and alcoholism, as this is likely to provide new molecular targets for clinical intervention.
- ^ Cite error: The named reference
pmid23920159was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Epidemwas invoked but never defined (see the help page). - ^ a b c d "AMERICAN BOARD OF MEDICAL SPECIALTIES RECOGNIZES THE NEW SUBSPECIALTY OF ADDICTION MEDICINE" (PDF). American Board of Addiction Medicine. 14 March 2016. Retrieved 3 April 2016.
Sixteen percent of the non-institutionalized U.S. population age 12 and over – more than 40 million Americans – meets medical criteria for addiction involving nicotine, alcohol or other drugs. This is more than the number of Americans with cancer, diabetes or heart conditions. In 2014, 22.5 million people in the United States needed treatment for addiction involving alcohol or drugs other than nicotine, but only 11.6 percent received any form of inpatient, residential, or outpatient treatment. Of those who do receive treatment, few receive evidence-based care. (There is no information available on how many individuals receive treatment for addiction involving nicotine.)
Risky substance use and untreated addiction account for one-third of inpatient hospital costs and 20 percent of all deaths in the United States each year, and cause or contribute to more than 100 other conditions requiring medical care, as well as vehicular crashes, other fatal and non-fatal injuries, overdose deaths, suicides, homicides, domestic discord, the highest incarceration rate in the world and many other costly social consequences. The economic cost to society is greater than the cost of diabetes and all cancers combined. Despite these startling statistics on the prevalence and costs of addiction, few physicians have been trained to prevent or treat it. - ^ a b c d Nora Volkow (31 March 2016). "A Major Step Forward for Addiction Medicine". National Institute on Drug Abuse. National Institutes of Health. Retrieved 3 April 2016.
Only about 10 percent of the 21 million Americans who meet the need for care for an alcohol or drug use disorder receive any form of treatment, and much of the treatment available does not meet standards for evidence-based care. There are many attitudinal and systemic reasons for this treatment gap, including stigma against treating people with addictions and institutional barriers to providing or funding addiction treatment. ... A major milestone was reached on March 14, 2016, when the American Board of Medical Specialties (ABMS) formally announced recognition of the field of Addiction Medicine as a medical subspecialty. ... In a statement issued to mark this milestone, ABAM President Robert J. Sokol summed up its significance: 'This landmark event, more than any other, recognizes addiction as a preventable and treatable disease, helping to shed the stigma that has long plagued it. It sends a strong message to the public that American medicine is committed to providing expert care for this disease and services designed to prevent the risky substance use that precedes it.'
- ^ Cite error: The named reference
Natural and drug addictionswas invoked but never defined (see the help page). - ^ Karila L, Wéry A, Weinstein A, Cottencin O, Petit A, Reynaud M, Billieux J (2014). "Sexual addiction or hypersexual disorder: different terms for the same problem? A review of the literature". Curr. Pharm. Des. 20 (25): 4012–4020. doi:10.2174/13816128113199990619. PMID 24001295.
Sexual addiction, which is also known as hypersexual disorder, has largely been ignored by psychiatrists, even though the condition causes serious psychosocial problems for many people. A lack of empirical evidence on sexual addiction is the result of the disease's complete absence from versions of the Diagnostic and Statistical Manual of Mental Disorders. ... Existing prevalence rates of sexual addiction-related disorders range from 3% to 6%. Sexual addiction/hypersexual disorder is used as an umbrella construct to encompass various types of problematic behaviors, including excessive masturbation, cybersex, pornography use, sexual behavior with consenting adults, telephone sex, strip club visitation, and other behaviors. The adverse consequences of sexual addiction are similar to the consequences of other addictive disorders. Addictive, somatic and psychiatric disorders coexist with sexual addiction. In recent years, research on sexual addiction has proliferated, and screening instruments have increasingly been developed to diagnose or quantify sexual addiction disorders. In our systematic review of the existing measures, 22 questionnaires were identified. As with other behavioral addictions, the appropriate treatment of sexual addiction should combine pharmacological and psychological approaches.
- ^ Cheetham A, Allen NB, Yücel M, Lubman DI (August 2010). "The role of affective dysregulation in drug addiction". Clin Psychol Rev. 30 (6): 621–34. doi:10.1016/j.cpr.2010.04.005. PMID 20546986.
- ^ Franken IH, Muris P (2006). "BIS/BAS personality characteristics and college students' substance use". Personality and Individual Differences. 40 (7): 1497–1503. doi:10.1016/j.paid.2005.12.005.
- ^ Genovese JE, Wallace D (December 2007). "Reward sensitivity and substance abuse in middle school and high school students". J Genet Psychol. 168 (4): 465–9. doi:10.3200/GNTP.168.4.465-469. PMID 18232522.
- ^ Kimbrel NA, Nelson-Gray RO, Mitchell JT (April 2007). "Reinforcement sensitivity and maternal style as predictors of psychopathology". Personality and Individual Differences. 42 (6): 1139–1149. doi:10.1016/j.paid.2006.06.028.
- ^ Dawe S, Loxton NJ (May 2004). "The role of impulsivity in the development of substance use and eating disorders". Neurosci Biobehav Rev. 28 (3): 343–51. doi:10.1016/j.neubiorev.2004.03.007. PMID 15225976.
External links
- "The Science of Addiction: Genetics and the Brain". learn.genetics.utah.edu. Learn.Genetics - University of Utah.
- Why do our brains get addicted? – a TEDMED 2014 talk by Nora Volkow, the director of the National Institute on Drug Abuse at NIH.
Kyoto Encyclopedia of Genes and Genomes signal transduction pathways: