Diphenoxylate: Difference between revisions
m →Related drugs: HTTP→HTTPS for NASA websites, per BRFA 8 using AWB |
→Inclusion of atropine: It cites two sources, but they might not be enough. |
||
| Line 69: | Line 69: | ||
==Inclusion of atropine== |
==Inclusion of atropine== |
||
| ⚫ | |||
Lomotil (diphenoxylate and atropine) contains diphenoxylate hydrochloride 2.5 mg, and atropine sulfate 0.025 mg to discourage deliberate overdosage and injection.<ref>http://www.rxlist.com/lomotil-drug.htm</ref> |
Lomotil (diphenoxylate and atropine) contains diphenoxylate hydrochloride 2.5 mg, and atropine sulfate 0.025 mg to discourage deliberate overdosage and injection.<ref>http://www.rxlist.com/lomotil-drug.htm</ref> |
||
| ⚫ | |||
The atropine additive strategy is designed to discourage use of the drug in a manner inconsistent with its labelling and physician and manufacturer instructions; in theory, the [[anticholinergic]] effect of atropine will produce severe weakness and nausea if standard dosage is exceeded, and at the time diphenoxylate was introduced in the United States a number of manufacturers used this strategy with oral forms of [[morphine]], [[methadone]] (also using [[Hyoscine hydrobromide|scopolamine]]), [[oxycodone]] and others. Currently, the only other narcotic produced in the United States to use this strategy is Motofen ([[difenoxin]] 1 mg with 25 µg atropine sulfate). Difenoxin is a DEA schedule C-I without atropine, but a schedule C-IV or C-V with varying doses of concurrent atropine.<ref>http://www.deadiversion.usdoj.gov/schedules/orangebook/a_sched_alpha.pdf</ref> |
The atropine additive strategy is designed to discourage use of the drug in a manner inconsistent with its labelling and physician and manufacturer instructions; in theory, the [[anticholinergic]] effect of atropine will produce severe weakness and nausea if standard dosage is exceeded, and at the time diphenoxylate was introduced in the United States a number of manufacturers used this strategy with oral forms of [[morphine]], [[methadone]] (also using [[Hyoscine hydrobromide|scopolamine]]), [[oxycodone]] and others. Currently, the only other narcotic produced in the United States to use this strategy is Motofen ([[difenoxin]] 1 mg with 25 µg atropine sulfate). Difenoxin is a DEA schedule C-I without atropine, but a schedule C-IV or C-V with varying doses of concurrent atropine.<ref>http://www.deadiversion.usdoj.gov/schedules/orangebook/a_sched_alpha.pdf</ref> |
||
Revision as of 06:39, 25 August 2017
This article possibly contains original research. (June 2011) |
This article needs additional citations for verification. (June 2011) |
 | |
 | |
| Clinical data | |
|---|---|
| Other names | R-1132, NIH-756 |
| AHFS/Drugs.com | Monograph |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 74-95% |
| Elimination half-life | 12–14 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.011.837 |
| Chemical and physical data | |
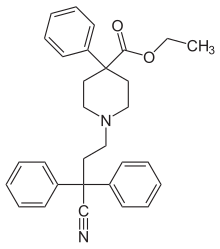
| Formula | C30H32N2O2 |
| Molar mass | 452.587 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Diphenoxylate (INN, BAN) (brand name Diocalm), or diphenoxylate hydrochloride (USAN), is a centrally active opioid drug of the phenylpiperidine series that is used for the treatment of diarrhea. It acts by slowing intestinal contractions and peristalsis allowing the body to consolidate intestinal contents and prolong transit time, thus allowing the intestines to draw moisture out of them at a normal or higher rate and therefore stop the formation of loose and liquid stools. It is the main active ingredient in the anti-peristaltic medication Lomotil, which also contains atropine.
Diphenoxylate is the prototype of a small subfamily of opioids (the phenylpiperidines) including difenoxin (diphenoxylic acid), an active metabolite of diphenoxylate produced in the human body, and loperamide, which unlike the former two works exclusively in the intestines because it only crosses the blood–brain barrier in very small amounts. It was discovered at Janssen Pharmaceutica in 1956. It is a congener to the narcotic pethidine. Other somewhat more distant relatives include alphaprodine and piritramide.
Actions and use
Diphenoxylate works by decreasing the speed and amplitude of wave-like movements of the intestines (peristalsis) therefore allowing the body time to remove moisture from the intestinal contents and consolidate waste product into a dense solid form rather than loose and watery as is diarrhea.
Similar to loperamide and difenoxin preparations, the usual protocol is to take a loading dose (usually two tablets or the quantity of liquid containing 5 mg of diphenoxylate) and then the standard dose of one tablet every 3 to 4 hours prn over a time period not to exceed 48 hours unless so ordered by the physician. Long-term intermittent prn users should contact their physician if the need for it arises more than was usual at the outset of therapy as tolerance to diphenoxylate can occur. Those taking it in large quantities or continuously over an extended period should taper down the dose on a schedule devised by the physician and/or pharmacist.
Related drugs
Diphenoxylate is metabolized in the body to yield difenoxin. Difenoxin is another centrally acting antidiarrheal that is 250 to 400 percent the strength of diphenoxylate via the oral route and is also able to be manufactured and is distributed as Motofen, (1 mg difenoxin/25 µg atropine), which was introduced in the United States in the late 1990s after being discovered in 1970 at Janssen.
The diphenoxylate/loperamide subfamily of gastrointestinal drugs works directly on opioid receptors, which can be found in the intestine, brain, and spinal cord. Diphenoxylate itself crosses the blood–brain barrier. This being the case, this medication is potentially habit-forming and can generate significant tolerance if taken continuously for a protracted period. Physical dependence is most common particularly with high doses and/or long-term use. The CNS penetration of diphenoxylate makes it an agent that can cause euphoria and other psychoactive effects, which could over time lead to habituation and dependency on the drug in the user. As with other medicinal opioids, iatrogenic addiction is uncommon although physical dependence secondary to treatment of a functional bowel disorder with diphenoxylate for more than 45–90 days may very well occur; it typically requires the use of high doses to impart a morbid seek orientation for the drug in the user. Because of this, diphenoxylate is manufactured and marketed as a combination drug with atropine (Lomotil, Pfizer) as an abuse deterrent. Lomotil was used during the Apollo program, as was pethidine.[1][2]
Inclusion of atropine
This section needs additional citations for verification. (August 2017) |
Lomotil (diphenoxylate and atropine) contains diphenoxylate hydrochloride 2.5 mg, and atropine sulfate 0.025 mg to discourage deliberate overdosage and injection.[3]
The atropine additive strategy is designed to discourage use of the drug in a manner inconsistent with its labelling and physician and manufacturer instructions; in theory, the anticholinergic effect of atropine will produce severe weakness and nausea if standard dosage is exceeded, and at the time diphenoxylate was introduced in the United States a number of manufacturers used this strategy with oral forms of morphine, methadone (also using scopolamine), oxycodone and others. Currently, the only other narcotic produced in the United States to use this strategy is Motofen (difenoxin 1 mg with 25 µg atropine sulfate). Difenoxin is a DEA schedule C-I without atropine, but a schedule C-IV or C-V with varying doses of concurrent atropine.[4]
The 25 µg of atropine present in each 2½ mg Lomotil tablet is 1/40 of the standard therapeutic dose of atropine via the oral route for normal anticholinergic uses.
Use in irritable bowel syndrome
Diarrhoea resulting from cyclic or diarrhoea-predominant IBS may not be optimally treated with diphenoxylate or difenoxin, and may not respond to a meaningful degree to loperamide; thus, diarrhoea and cramping which does not respond to belladonna derivatives and non-centrally-acting soothing and/or stool-desiccating agents are often treated with conservative doses of codeine, especially where paregoric and/or laudanum are not currently in general use.
Diarrhoea accompanied by significant cramping or intestinal cramping alone may benefit from either adding another smooth-muscle agent to the protocol such as dicycloverine, papaverine, or orphenadrine (which accomplishes several things at once since it is an antihistamine as noted above). Such cases may also benefit from a switch to paregoric, laudanum, powdered or granulated opium, or belladonna-and-opium suppositories, as all of the above include many drugs thatwork together and have non-narcotic alkaloids like papaverine and other components like oils, waxes, resins etc. which work elsewhere in the body. Pantopon (opium alkaloids hydrochlorides) and similar preparations have much of the advantages of whole opium in injectable form.
Regulation
Diphenoxylate is listed in national controlled-substances and drugs laws such as pure diphenoxylate being in Annex/Schedule/List II of the Single Convention on Narcotic Drugs of 1961 and also in similarly numbered schedules of laws passed to implement the Convention such as the Canadian Controlled Substances Act and the Betäubungsmittelgesetz in Germany and the Suchtgiftverordnung of Austria. This level of regulation is also implemented in the Misuse of Drugs Act of 1971 (UK), the French opium law of 31 December 1970, and royal decrees and laws passed by practically all other countries in Europe, the Pacific Rim, South Asia and the Middle East.
In many cases, Lomotil type preparations with extra active ingredients are over the counter and/or subject to provincial and/or municipal control or minimal national oversight; the schedule numbers vary by country.
This medication is classified as a Schedule V under the United States' Comprehensive Drug Abuse Control & Prevention Act of 1970 aka Controlled Substances Act (CSA) (21 U.S.C. § 801 et. seq.) by the Food and Drug Administration (FDA) and the Drug Enforcement Administration (DEA) in the United States when used in preparations. When diphenoxylate is used alone, it is classified as a Schedule II; having one or more other active ingredients makes it Schedule V. As such, diphenoxylate/atropine tablets are under state and local control and where allowed by state law, can be obtained by signing a log book and the customer can receive up to 48 units (tablets or capsules) in any 48-hour period, much like the 4 fl. oz. limit on codeine, dihydrocodeine, dionine (ethylmorphine), and opium cough syrups and gastrointestinal drugs. Bulk diphenoxylate (Schedule II) has a DEA ACSCN of 9170 and an annual aggregate manufacturing quota of 750 kilos as of 2013.
In the United States, difenoxin and atropine tablets are Schedule IV and therefore federally controlled and require a prescription, loperamide is unscheduled and not on prescription, and diphenoxylic acid is classified as a form of diphenoxylate. Other Schedule IV narcotics also include butorphanol nasal spray, pentazocine, propoxyphene-based mixtures and others of the same type. Unscheduled narcotics and opioid agonists include loperamide (OTC), and nalbuphine (Rx).
Lomotil is a Schedule V drug, but it is only available by prescription in all 50 of the United States on account of the atropine content from 1. January 1993. Likewise, Donnagel-PG: was available without prescription in some states, but went on prescription in 1993 and later was discontinued by the manufacturer although compounding pharmacies have continued to make it when requested by the doctor. The stimulant pyrovalerone is another Schedule V prescription-only drug (not marketed), as is Lyrica, a relative of gabapentin used for chronic neuropathic pain and other conditions.[5]
References
- Merlo M, Brown CH, The effect of diphenoxylate hydrochloride on diarrhea, Am J Gastroenterol. 1960 Dec;34:625-30.
- Kasic AM, Treatment of diarrhea in irritable colon, including preliminary observations with a new antidiarrheal agent, diphenoxylate hydrochloride (Lomotil), Am J Gastroenterol. 1961 Jan;35:46-9.
