Iso E Super: Difference between revisions
KolbertBot (talk | contribs) m Bot: HTTP→HTTPS (v477) |
No edit summary Tag: references removed |
||
| Line 24: | Line 24: | ||
| RefractIndex = 1.4975–1.500 (at 20 °C) |
| RefractIndex = 1.4975–1.500 (at 20 °C) |
||
| Odor = amber, woody |
| Odor = amber, woody |
||
| LogP = 5.65 |
|||
| Solubility = }} |
| Solubility = }} |
||
|Section3={{Chembox Hazards |
|Section3={{Chembox Hazards |
||
| Line 46: | Line 47: | ||
Iso E Super may cause allergic reactions detectable by [[patch test]]s in humans<ref name="derm">{{citation | author=PJ Frosch | title=Patch testing with fragrances: results of a multicenter study of the European Environmental and Contact Dermatitis Research Group with 48 frequently used constituents of perfumes. | journal=Contact Dermatitis | volume=33 | issue=5 |date=Nov 1995 | pages=333–42 | pmid=8565489 | doi=10.1111/j.1600-0536.1995.tb02048.x|display-authors=etal}}</ref> and chronic exposure to Iso E Super from perfumes may result in permanent [[hypersensitivity]].<ref name="aqua1"/> In a study with female mice, Iso E Super was positive in the [[local lymph node assay]] (LLNA) and [[irritancy assay]] (IRR), but negative in the [[mouse ear swelling test]] (MEST).<ref name="mice">{{citation | title=NTP Report on the Assessment of Contact Hypersensitivity to Iso-E Super in Female BALB/c Mice (CASRN: 54464-57-2) | publisher=National Institute of Environmental Health Sciences | year=2010 | url=http://ntp.niehs.nih.gov/?objectid=FD21FC74-C672-AC9C-5395E19CB7EB1F04 | deadurl=yes | archiveurl=https://web.archive.org/web/20140221071404/http://ntp.niehs.nih.gov/?objectid=FD21FC74-C672-AC9C-5395E19CB7EB1F04 | archivedate=2014-02-21 | df= }}</ref> |
Iso E Super may cause allergic reactions detectable by [[patch test]]s in humans<ref name="derm">{{citation | author=PJ Frosch | title=Patch testing with fragrances: results of a multicenter study of the European Environmental and Contact Dermatitis Research Group with 48 frequently used constituents of perfumes. | journal=Contact Dermatitis | volume=33 | issue=5 |date=Nov 1995 | pages=333–42 | pmid=8565489 | doi=10.1111/j.1600-0536.1995.tb02048.x|display-authors=etal}}</ref> and chronic exposure to Iso E Super from perfumes may result in permanent [[hypersensitivity]].<ref name="aqua1"/> In a study with female mice, Iso E Super was positive in the [[local lymph node assay]] (LLNA) and [[irritancy assay]] (IRR), but negative in the [[mouse ear swelling test]] (MEST).<ref name="mice">{{citation | title=NTP Report on the Assessment of Contact Hypersensitivity to Iso-E Super in Female BALB/c Mice (CASRN: 54464-57-2) | publisher=National Institute of Environmental Health Sciences | year=2010 | url=http://ntp.niehs.nih.gov/?objectid=FD21FC74-C672-AC9C-5395E19CB7EB1F04 | deadurl=yes | archiveurl=https://web.archive.org/web/20140221071404/http://ntp.niehs.nih.gov/?objectid=FD21FC74-C672-AC9C-5395E19CB7EB1F04 | archivedate=2014-02-21 | df= }}</ref> |
||
Iso E Super is [[toxic]] and [[bioaccumulative]] in aquatic organisms and the environment, and is suspected to be bioaccumulative in humans. |
|||
Iso E Super is [[toxic]] and [[bioaccumulative]] in aquatic organisms and the environment,<ref name="aqua1">{{citation | title=Iso E Super Material Safety Data Sheet | publisher=Santa Cruz Biotechnology | url=http://datasheets.scbt.com/sc-211663.pdf}}</ref><ref name="aqua2">{{citation | title=Predicting the Bioconcentration of Fragrance Ingredients by Rainbow Trout Using Measured Rates of in Vitro Intrinsic Clearance | publisher=ACS Publications, Environmental Science & Technology | year=2014 | url=http://www.d.umn.edu/biology/documents/Nichols1.pdf}}</ref> and is suspected to be bioaccumulative in humans.<ref name="bioacc">{{citation | title=Ranking of Concern from persistence, bioaccumulation and toxicity in the environmentof Pharmaceuticals and Personal Care Products | url=https://www.researchgate.net/profile/R_Irusta/publication/266516858_Ranking_of_Concern_from_persistence_bioaccumulation_and_toxicity_in_the_environment_of_Pharmaceuticals_and_Personal_Care_Products/links/5433d2780cf2bf1f1f263502.pdf}}</ref> |
|||
No data were available regarding chemical disposition, metabolism, or toxicokinetics; acute, shortterm, subchronic, or chronic toxicity; synergistic or antagonistic activity; reproductive or teratological effects; carcinogenicity; genotoxicity; or immunotoxicity.<ref name="tox" /> |
No data were available regarding chemical disposition, metabolism, or toxicokinetics; acute, shortterm, subchronic, or chronic toxicity; synergistic or antagonistic activity; reproductive or teratological effects; carcinogenicity; genotoxicity; or immunotoxicity.<ref name="tox" /> |
||
The [[International Fragrance Association]] (IFRA) has published safe use levels for Iso E Super in consumer products.<ref>http://www.ifraorg.org/en-us/standards-library/s/OTNE</ref> |
|||
== List of products containing Iso E Super == |
== List of products containing Iso E Super == |
||
Revision as of 13:36, 12 January 2018
It has been suggested that this article be merged with Tetramethyl_acetyloctahydronaphthalenes. (Discuss) Proposed since March 2016. |
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
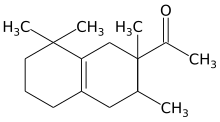
1-(1,2,3,4,5,6,7,8-octahydro-2,3,8,8,-tetramethyl-2-naphthyl)ethan-1-one
| |||
| Other names
Amberonne; Ambralux; Boisvelone; Derambrene; Timbersilk; Methyl cyclomyrectone; 1,2,3,4,5,6,7,8-octahydro-2,3,8,8-tetramethyl-2-acetonaphthalenone; OTNE
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ECHA InfoCard | 100.053.777 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C16H26O | |||
| Molar mass | 234.38 g/mol | ||
| Appearance | colorless to a pale yellow liquid | ||
| Odor | amber, woody | ||
| Boiling point | 134 °C (273 °F; 407 K) | ||
| log P | 5.65 | ||
Refractive index (nD)
|
1.4975–1.500 (at 20 °C) | ||
| Hazards | |||
| Flash point | > 100 °C (closed cup) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Iso E Super is a synthetic ketone fragrance.
Uses
Iso E Super is a very common perfume ingredient, providing a sandalwood-like and cedarwood-like fragrance, in soap, shampoo, perfumes, detergents, fabric fresheners, antiperspirants or deodorants, and air fresheners. It is also used as a tobacco flavoring (at 200–2000 ppm), as a plasticizer and as a precursor for the delivery of organoleptic and antimicrobial compounds.[1]
Production
Iso E Super is produced commercially by Diels–Alder reaction of Myrcene with 3-Methyl-3-penten-2-one in the presence of aluminum chloride to give a monocyclic intermediate that is cyclized in the presence of 85% phosphoric acid.[2]

Carrying out the initial Diels-Alder reaction using a Lewis acid catalyst such as aluminum chloride appears to ensure that the acetyl group is at position 2 of the resulting cyclohexene adduct, which distinguished Iso E Super from other (previously patented) fragrances based on tetramethyl-acetyl-octaline. The second cyclization reaction yields a mixture of diastereomers with the general structure depicted above, the predominant ones being (2R,3R) and (2S,3S).[3]
Safety
Iso E Super may cause allergic reactions detectable by patch tests in humans[4] and chronic exposure to Iso E Super from perfumes may result in permanent hypersensitivity.[5] In a study with female mice, Iso E Super was positive in the local lymph node assay (LLNA) and irritancy assay (IRR), but negative in the mouse ear swelling test (MEST).[6]
Iso E Super is toxic and bioaccumulative in aquatic organisms and the environment, and is suspected to be bioaccumulative in humans.
No data were available regarding chemical disposition, metabolism, or toxicokinetics; acute, shortterm, subchronic, or chronic toxicity; synergistic or antagonistic activity; reproductive or teratological effects; carcinogenicity; genotoxicity; or immunotoxicity.[1]
The International Fragrance Association (IFRA) has published safe use levels for Iso E Super in consumer products.[7]
List of products containing Iso E Super
- The fragrance Molecule 01 (Escentric Molecules, 2005) is essentially just Iso E Super note.[8] Its partner fragrance Escentric 01 contains Iso E Super but also ambroxan, pink pepper, green lime with balsamic notes like benzoin mastic and incense.
- The fragrance Eternity by Calvin Klein (1988) contained 11.7% Iso E Super in the fragrance portion of the formula.[1]
- The fragrance "Paper " by Commodity, lists Iso E Super in the fragrance description on the website.
- The male fragrance Fahrenheit (Dior, 1988) is 25% Iso E Super. (of the fragrance compound).
- While Lalique's Eau de Parfum Perles de Lalique (Lalique, 2007) is apparently a whopping 80% Iso E Super (of the perfume compound).
- The men's fragrance Encre Noire (Lalique, 2006) is 45% of the fragrance compound, Iso E Super.
- The fragrance Poivre Samarcande (Hermes, 2004) contains 71% Iso E Super (of the perfume compound).
- The very popular Terre D'Hermes (Hermes, 2006) contains 55% Iso E Super (of the perfume compound).
- Kenzo Air (Kenzo, 2003) is 48% of the fragrance compound Iso E Super.
- The men's fragrance Fierce Cologne (Abercrombie & Fitch, 2002) is 48% Iso E Super.
- Home fragance "Under a fig tree" from Rituals brand.
- Creed's Aventus (2010) contains 18% Iso E Super in its fragrance compound.
References
- ^ a b c Bonnie L. Carson (2001), 1-(1,2,3,4,5,6,7,8-Octahydro-2,3-8,8-tetramethyl-2-naphthalenyl) ethanone. Review of Toxicological Literature (PDF), National Institute of Environmental Health Sciences
- ^ Karl-Georg Fahlbusch; et al. (2007), "Flavors and Fragrances", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, pp. 45–46
- ^ Perfume composition and perfume articles containing one isomer of an octahydrotetramethyl acetonaphthone
{{citation}}: Unknown parameter|country-code=ignored (help); Unknown parameter|inventor1-first=ignored (help); Unknown parameter|inventor1-last=ignored (help); Unknown parameter|inventor2-first=ignored (help); Unknown parameter|inventor2-last=ignored (help); Unknown parameter|issue-date=ignored (help); Unknown parameter|patent-number=ignored (help) - ^ PJ Frosch; et al. (Nov 1995), "Patch testing with fragrances: results of a multicenter study of the European Environmental and Contact Dermatitis Research Group with 48 frequently used constituents of perfumes.", Contact Dermatitis, 33 (5): 333–42, doi:10.1111/j.1600-0536.1995.tb02048.x, PMID 8565489
- ^ Cite error: The named reference
aqua1was invoked but never defined (see the help page). - ^ NTP Report on the Assessment of Contact Hypersensitivity to Iso-E Super in Female BALB/c Mice (CASRN: 54464-57-2), National Institute of Environmental Health Sciences, 2010, archived from the original on 2014-02-21
{{citation}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ http://www.ifraorg.org/en-us/standards-library/s/OTNE
- ^ "Stagnant water, charcoal, semen... 10 smells that changed the perfume industry". The Telegraph. Retrieved 2017-06-21.


