Tetrazolium chloride: Difference between revisions
GoingBatty (talk | contribs) m →TTC assay: clean up, typo(s) fixed: in it's → in its using AWB |
|||
| Line 60: | Line 60: | ||
In the TTC assay (also known as TTC test or tetrazolium test), TTC is used to differentiate between metabolically active and inactive tissues. The white compound is enzymatically reduced to red TPF (1,3,5-triphenylformazan) in living tissues due to the activity of various dehydrogenases (enzymes important in oxidation of organic compounds and thus cellular metabolism), while it remains in its unreacted state in areas of necrosis since these enzymes have either denatured or degraded. |
In the TTC assay (also known as TTC test or tetrazolium test), TTC is used to differentiate between metabolically active and inactive tissues. The white compound is enzymatically reduced to red TPF (1,3,5-triphenylformazan) in living tissues due to the activity of various dehydrogenases (enzymes important in oxidation of organic compounds and thus cellular metabolism), while it remains in its unreacted state in areas of necrosis since these enzymes have either denatured or degraded. |
||
TTC has been employed in autopsy pathology to assist post-mortem identification of myocardial infarctions. Healthy viable heart muscle will stain deep red from the cardiac lactate dehydrogenase |
TTC has been employed in autopsy pathology to assist post-mortem identification of myocardial infarctions. Healthy viable heart muscle will stain deep red from the cardiac lactate dehydrogenase, while areas of potential infarctions will be more pale. |
||
[[File:Ttc.PNG|500px]] |
[[File:Ttc.PNG|500px]] |
||
Revision as of 12:41, 19 January 2018
| |

| |
| Names | |
|---|---|
| IUPAC name
2,3,5-Triphenyl-2H-tetrazolium chloride
| |
| Other names
TTC
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.005.520 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H15ClN4 | |
| Molar mass | 334.8 g/mol |
| Appearance | white crystalline powder |
| Soluble | |
| Solubility in water | ~1 mg/ml |
| Solubility in PBS (pH 7.2) | ~1 mg/ml |
| Solubility in ethanol | ~1 mg/ml |
| Solubility in DMSO | ~0.25 mg/ml |
| log P | −2.4 |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H228 | |
| P210 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
5.6 mg/kg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
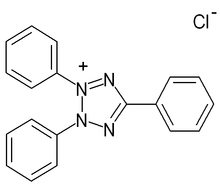
Triphenyl tetrazolium chloride, TTC, or simply tetrazolium chloride (with the formula 2,3,5-triphenyl-2H-tetrazolium chloride) is a redox indicator commonly used in biochemical experiments especially to indicate cellular respiration. It is a white crystalline powder, soluble in water, ethanol and acetone but insoluble in ether.[1][2]
TTC assay
In the TTC assay (also known as TTC test or tetrazolium test), TTC is used to differentiate between metabolically active and inactive tissues. The white compound is enzymatically reduced to red TPF (1,3,5-triphenylformazan) in living tissues due to the activity of various dehydrogenases (enzymes important in oxidation of organic compounds and thus cellular metabolism), while it remains in its unreacted state in areas of necrosis since these enzymes have either denatured or degraded.
TTC has been employed in autopsy pathology to assist post-mortem identification of myocardial infarctions. Healthy viable heart muscle will stain deep red from the cardiac lactate dehydrogenase, while areas of potential infarctions will be more pale.
See also
References
- ^ Witty M. (2012). Topographischer Nachweis der Keimfähigkeit der Getreidefrüchte durch Tetrazoliumsalze (Topographic Detection of Germination in Cereal Crops by Tetrazolium Salts) — A Translation of Lakon’s 1942 Paper on Tetrazolium Seed Testing. Seed Technology 34(2):275-282.
- ^ Witty M. (2012). The process for 2, 3, 5 - triphenyl – tetrazolium chloride synthesis, an intellectual property seized immediately after world war II. Bulletin for the History of Chemistry 37(2):91-95.


