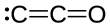Dicarbon monoxide: Difference between revisions
Context Tags: Mobile edit Mobile web edit |
m Undid revision 827907910 by Jithin krishna (talk) |
||
| Line 34: | Line 34: | ||
==Occurrence== |
==Occurrence== |
||
Dicarbon monoxide |
Dicarbon monoxide is a product of the [[Photodissociation|photolysis]] of [[carbon suboxide]]:<ref>{{ cite journal | author= Bayes, K. | title = Photolysis of Carbon Suboxide | journal = [[Journal of the American Chemical Society]] | volume = 83 | year = 1961 | issue = 17 | pages = 3712–3713 | doi = 10.1021/ja01478a033 }}</ref><ref>{{ cite journal |author1=Anderson, D. J. |author2=Rosenfeld, R. N. | title = Photodissociation of Carbon Suboxide | journal = [[Journal of Chemical Physics]] | volume = 94 | year = 1991 | issue = 12 | pages = 7857–7867 | doi = 10.1063/1.460121 }}</ref> |
||
:C<sub>3</sub>O<sub>2</sub> → CO + C<sub>2</sub>O |
:C<sub>3</sub>O<sub>2</sub> → CO + C<sub>2</sub>O |
||
Revision as of 14:50, 27 February 2018
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Oxoethenylidene
| |||
| Other names
Ketenylidene
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
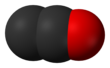
| C2O | |||
| Molar mass | 40.021 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Dicarbon monoxide (C2O) is molecule that contains two carbon atoms and one oxygen atom. It is a linear molecule that, because of its simplicity, is of interest in a variety of areas. It is, however, so extremely reactive that it is not encountered in everyday life. It is classified as a cumulene and an oxocarbon.[1]
Occurrence
Dicarbon monoxide is a product of the photolysis of carbon suboxide:[2][3]
- C3O2 → CO + C2O
It is stable enough to observe reactions with NO and NO2.[4]
Called ketenylidene in organometallic chemistry, it is a ligand observed in metal carbonyl clusters, e.g. [OC2Co3(CO)9]+. Ketenylidenes are proposed as intermediates in the chain growth mechanism of the Fischer-Tropsch Process, which converts carbon monoxide and hydrogen to hydrocarbon fuels.[5]
The organophosphorus compound (C6H5)3PCCO (CAS# 15596-07-3) contains the C2O functionality. Sometimes called Bestmann's Ylide, it is a yellow solid.[6]
References
- ^ Frenking, Gernot; Tonner, Ralf "Divalent carbon(0) compounds" Pure and Applied Chemistry 2009, vol. 81, pp. 597-614. doi:10.1351/PAC-CON-08-11-03
- ^ Bayes, K. (1961). "Photolysis of Carbon Suboxide". Journal of the American Chemical Society. 83 (17): 3712–3713. doi:10.1021/ja01478a033.
- ^ Anderson, D. J.; Rosenfeld, R. N. (1991). "Photodissociation of Carbon Suboxide". Journal of Chemical Physics. 94 (12): 7857–7867. doi:10.1063/1.460121.
- ^ Thweatt, W. D.; Erickson, M. A.; Hershberger, J. F. (2004). "Kinetics of the CCO + NO and CCO + NO2 reactions". Journal of Physical Chemistry A. 108 (1): 74–79. doi:10.1021/jp0304125.
- ^ Jensen, Michael P.; Shriver, Duward F. "Carbon-carbon and carbonyl transformations in ketenylidene cluster compounds" Journal of Molecular Catalysis 1992, vol. 74, pp. 73-84. doi:10.1016/0304-5102(92)80225-6
- ^ H. J. Bestmann, R. Zimmermann, M. Riou "Ketenylidenetriphenylphosphorane" e-EROS Encyclopedia of Reagents for Organic Synthesis 2001. doi:10.1002/047084289X.rk005.pub2