Rhodium(III) oxide: Difference between revisions
→Production: another lab prep |
Rescuing 0 sources and tagging 2 as dead. #IABot (v1.6.5) |
||
| Line 52: | Line 52: | ||
==Physical properties== |
==Physical properties== |
||
Rhodium oxide films behave as a fast two-color [[Electrochromism|electrochromic]] system: Reversible yellow ↔ dark green or yellow ↔ brown-purple color changes are obtained in [[potassium hydroxide|KOH]] solutions by applying voltage ~1 [[volt|V]].<ref>S. Gottesfeld "The Anodic Rhodium Oxide Film: A Two-Color |
Rhodium oxide films behave as a fast two-color [[Electrochromism|electrochromic]] system: Reversible yellow ↔ dark green or yellow ↔ brown-purple color changes are obtained in [[potassium hydroxide|KOH]] solutions by applying voltage ~1 [[volt|V]].<ref>S. Gottesfeld "The Anodic Rhodium Oxide Film: A Two-Color |
||
Electrochromic System" [http://link.aip.org/link/?JESOAN/127/272/1 J. Electrochem. Soc. 127 (1980) 272]</ref> |
Electrochromic System" [http://link.aip.org/link/?JESOAN/127/272/1 J. Electrochem. Soc. 127 (1980) 272]{{dead link|date=April 2018 |bot=InternetArchiveBot |fix-attempted=yes }}</ref> |
||
Rhodium oxide films are transparent and conductive, like [[indium tin oxide]] (ITO) - the common transparent electrode, but Rh<sub>2</sub>O<sub>3</sub> has 0.2 eV lower [[work function]] than ITO. Consequently, deposition of rhodium oxide on ITO improves the carrier injection from ITO thereby improving the electrical properties of [[organic light-emitting diode]]s.<ref name=apl>S. Y. Kim et al. "Rhodium-oxide-coated indium tin oxide for enhancement of hole injection in organic light emitting diodes" [http://link.aip.org/link/?APPLAB/87/072105/1 Appl. Phys. Lett. 87 (2005) 072105 ]</ref> |
Rhodium oxide films are transparent and conductive, like [[indium tin oxide]] (ITO) - the common transparent electrode, but Rh<sub>2</sub>O<sub>3</sub> has 0.2 eV lower [[work function]] than ITO. Consequently, deposition of rhodium oxide on ITO improves the carrier injection from ITO thereby improving the electrical properties of [[organic light-emitting diode]]s.<ref name=apl>S. Y. Kim et al. "Rhodium-oxide-coated indium tin oxide for enhancement of hole injection in organic light emitting diodes" [http://link.aip.org/link/?APPLAB/87/072105/1 Appl. Phys. Lett. 87 (2005) 072105 ]{{dead link|date=April 2018 |bot=InternetArchiveBot |fix-attempted=yes }}</ref> |
||
==Applications== |
==Applications== |
||
Revision as of 12:15, 16 April 2018
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ECHA InfoCard | 100.031.666 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Rh2O3 | |
| Molar mass | 253.8092 g/mol |
| Appearance | dark grey odorless powder |
| Density | 8.20 g/cm3 |
| Melting point | 1,100 °C (2,010 °F; 1,370 K) (decomposes) |
| insoluble | |
| Solubility | insoluble in aqua regia |
| +104.0·10−6 cm3/mol | |
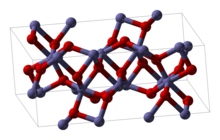
| Structure | |
| hexagonal (corundum) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Rhodium(III) oxide (or Rhodium sesquioxide) is the inorganic compound with the formula Rh2O3. It is a gray solid that is insoluble in ordinary solvents.
Structure
Rh2O3 has been found in two major forms. The hexagonal form adopts the corundum structure. It transforms into an orthorhombic structure when heated above 750 °C.[1]
Production
Rhodium oxide can be produced via several routes:
- Treating RhCl3 with oxygen at high temperatures.[2]
- Rh metal powder is fused with potassium hydrogen sulfate. Adding sodium hydroxide results in hydrated rhodium oxide, which upon heating converts to Rh2O3.[3]
- Rhodium oxide thin films can be produced by exposing Rh layer to oxygen plasma.[4]
- Nanoparticles can be produced by the hydrothermal synthesis.[5]
Physical properties
Rhodium oxide films behave as a fast two-color electrochromic system: Reversible yellow ↔ dark green or yellow ↔ brown-purple color changes are obtained in KOH solutions by applying voltage ~1 V.[6]
Rhodium oxide films are transparent and conductive, like indium tin oxide (ITO) - the common transparent electrode, but Rh2O3 has 0.2 eV lower work function than ITO. Consequently, deposition of rhodium oxide on ITO improves the carrier injection from ITO thereby improving the electrical properties of organic light-emitting diodes.[4]
Applications
The major application of rhodium oxides is in catalysts (e.g. hydroformylation reactions,[7] N2O production from NO,[8] or the hydrogenation of CO).[9]
See also
References
- ^ J. M. D. Coey "The crystal structure of Rh2O3" Acta Crystallogr. (1970). B26, 1876
- ^ H. L. Grube (1963). "The Platinum Metals". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. NY: Academic Press. p. 1588.
- ^ A. Wold et al. "The Reaction of Rare Earth Oxides with a High Temperature Form of Rhodium(III) Oxide" Inorg. Chem. 2 (1963) 972
- ^ a b S. Y. Kim et al. "Rhodium-oxide-coated indium tin oxide for enhancement of hole injection in organic light emitting diodes" Appl. Phys. Lett. 87 (2005) 072105 [permanent dead link]
- ^ R. S. Mulukutla "Synthesis and characterization of rhodium oxide nanoparticles in mesoporous MCM-41" Phys. Chem. Chem. Phys. 1 (1999) 2027
- ^ S. Gottesfeld "The Anodic Rhodium Oxide Film: A Two-Color Electrochromic System" J. Electrochem. Soc. 127 (1980) 272[permanent dead link]
- ^ Pino, P.; Botteghi, C. (1977). "Aldehydes from olefins: cyclohexanecarboxaldehyde". Organic Syntheses. 57: 11. doi:10.15227/orgsyn.057.0011.
- ^ R. S. Mulukutla "Characterization of rhodium oxide nanoparticles in MCM-41 and their catalytic performances for NO–CO reactions in excess O2" Applied Catalysis A: 228 (2002) 305
- ^ P. R. Watson and G. A. Somorjai "The hydrogenation of carbon monoxide over rhodium oxide surfaces" Journal of Catalysis 72 (1981) 347
