Glyceollin I: Difference between revisions
Appearance
Content deleted Content added
m BR separated pieces interpreted as multiple names re-joined with WBR |
Added free to read link in citations with OAbot #oabot |
||
| Line 44: | Line 44: | ||
[[Glyceollin synthase]] is an enzyme responsible for the production of glyceollin.<ref>{{Cite journal | last1 = Welle | first1 = R. | last2 = Grisebach | first2 = H. | doi = 10.1016/0003-9861(88)90627-3 | title = Induction of phytoalexin synthesis in soybean: Enzymatic cyclization of prenylated pterocarpans to glyceollin isomers | journal = Archives of Biochemistry and Biophysics | volume = 263 | issue = 1 | pages = 191–198 | year = 1988 | pmid = 3369863| pmc = }}</ref> The five substrates of this enzyme are 2-dimethylallyl-(6aS,11aS)-3,6a,9-trihydroxypterocarpan, 4-dimethylallyl-(6aS,11aS)-3,6a,9-trihydroxypterocarpan, [[nicotinamide adenine dinucleotide phosphate|NADPH]], [[hydrogen ion|H<sup>+</sup>]], and [[oxygen|O<sub>2</sub>]], whereas its three products are glyceollin, [[nicotinamide adenine dinucleotide phosphate|NADP<sup>+</sup>]], and [[water|H<sub>2</sub>O]]. |
[[Glyceollin synthase]] is an enzyme responsible for the production of glyceollin.<ref>{{Cite journal | last1 = Welle | first1 = R. | last2 = Grisebach | first2 = H. | doi = 10.1016/0003-9861(88)90627-3 | title = Induction of phytoalexin synthesis in soybean: Enzymatic cyclization of prenylated pterocarpans to glyceollin isomers | journal = Archives of Biochemistry and Biophysics | volume = 263 | issue = 1 | pages = 191–198 | year = 1988 | pmid = 3369863| pmc = }}</ref> The five substrates of this enzyme are 2-dimethylallyl-(6aS,11aS)-3,6a,9-trihydroxypterocarpan, 4-dimethylallyl-(6aS,11aS)-3,6a,9-trihydroxypterocarpan, [[nicotinamide adenine dinucleotide phosphate|NADPH]], [[hydrogen ion|H<sup>+</sup>]], and [[oxygen|O<sub>2</sub>]], whereas its three products are glyceollin, [[nicotinamide adenine dinucleotide phosphate|NADP<sup>+</sup>]], and [[water|H<sub>2</sub>O]]. |
||
In in vitro studies, this molecule has been shown to exhibit [[antiestrogenic]] properties.<ref>{{Cite journal | last1 = Payton-Stewart | first1 = F. | last2 = Khupse | first2 = R. S. | last3 = Boué | first3 = S. M. | last4 = Elliott | first4 = S. | last5 = Zimmermann | first5 = M. C. | last6 = Skripnikova | first6 = E. V. | last7 = Ashe | first7 = H. | last8 = Tilghman | first8 = S. L. | last9 = Beckman | first9 = B. S. | doi = 10.1016/j.steroids.2010.05.007 | last10 = Cleveland | first10 = T. E. | last11 = McLachlan | first11 = J. A. | last12 = Bhatnagar | first12 = D. | last13 = Wiese | first13 = T. E. | last14 = Erhardt | first14 = P. | last15 = Burow | first15 = M. E. | title = Glyceollin I enantiomers distinctly regulate ER-mediated gene expression | journal = Steroids | volume = 75 | issue = 12 | pages = 870–878 | year = 2010 | pmid = 20493896| pmc = }}</ref> |
In in vitro studies, this molecule has been shown to exhibit [[antiestrogenic]] properties.<ref>{{Cite journal | last1 = Payton-Stewart | first1 = F. | last2 = Khupse | first2 = R. S. | last3 = Boué | first3 = S. M. | last4 = Elliott | first4 = S. | last5 = Zimmermann | first5 = M. C. | last6 = Skripnikova | first6 = E. V. | last7 = Ashe | first7 = H. | last8 = Tilghman | first8 = S. L. | last9 = Beckman | first9 = B. S. | doi = 10.1016/j.steroids.2010.05.007 | last10 = Cleveland | first10 = T. E. | last11 = McLachlan | first11 = J. A. | last12 = Bhatnagar | first12 = D. | last13 = Wiese | first13 = T. E. | last14 = Erhardt | first14 = P. | last15 = Burow | first15 = M. E. | title = Glyceollin I enantiomers distinctly regulate ER-mediated gene expression | journal = Steroids | volume = 75 | issue = 12 | pages = 870–878 | year = 2010 | pmid = 20493896| pmc = | url = https://naldc.nal.usda.gov/naldc/download.xhtml?id=44156&content=PDF }}</ref> |
||
== References == |
== References == |
||
Revision as of 09:15, 29 April 2018
| |
| Names | |
|---|---|
| IUPAC name
(6aS,11aS)-2,2-dimethyl-2H,6H-[1]benzofuro[3,2‑c]pyrano[2,3‑h]
| |
| Other names
(−)-Glyceollin I
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.222.666 |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H18O5 | |
| Molar mass | 338 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
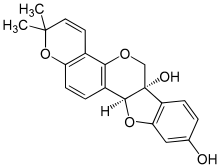
Glyceollin I is a glyceollin, a type of prenylated pterocarpan. It is a phytoalexin found in the soybean.[1]
Glyceollin synthase is an enzyme responsible for the production of glyceollin.[2] The five substrates of this enzyme are 2-dimethylallyl-(6aS,11aS)-3,6a,9-trihydroxypterocarpan, 4-dimethylallyl-(6aS,11aS)-3,6a,9-trihydroxypterocarpan, NADPH, H+, and O2, whereas its three products are glyceollin, NADP+, and H2O.
In in vitro studies, this molecule has been shown to exhibit antiestrogenic properties.[3]
References
- ^ Zimmermann, M. C.; Tilghman, S. L.; Boué, S. M.; Salvo, V. A.; Elliott, S.; Williams, K. Y.; Skripnikova, E. V.; Ashe, H.; Payton-Stewart, F.; Vanhoy-Rhodes, L.; Fonseca, J. P.; Corbitt, C.; Collins-Burow, B. M.; Howell, M. H.; Lacey, M.; Shih, B. Y.; Carter-Wientjes, C.; Cleveland, T. E.; McLachlan, J. A.; Wiese, T. E.; Beckman, B. S.; Burow, M. E. (2009). "Glyceollin I, a Novel Antiestrogenic Phytoalexin Isolated from Activated Soy". Journal of Pharmacology and Experimental Therapeutics. 332 (1): 35–45. doi:10.1124/jpet.109.160382. PMC 2802480. PMID 19797619.
- ^ Welle, R.; Grisebach, H. (1988). "Induction of phytoalexin synthesis in soybean: Enzymatic cyclization of prenylated pterocarpans to glyceollin isomers". Archives of Biochemistry and Biophysics. 263 (1): 191–198. doi:10.1016/0003-9861(88)90627-3. PMID 3369863.
- ^ Payton-Stewart, F.; Khupse, R. S.; Boué, S. M.; Elliott, S.; Zimmermann, M. C.; Skripnikova, E. V.; Ashe, H.; Tilghman, S. L.; Beckman, B. S.; Cleveland, T. E.; McLachlan, J. A.; Bhatnagar, D.; Wiese, T. E.; Erhardt, P.; Burow, M. E. (2010). "Glyceollin I enantiomers distinctly regulate ER-mediated gene expression". Steroids. 75 (12): 870–878. doi:10.1016/j.steroids.2010.05.007. PMID 20493896.
