Pyrosilicic acid: Difference between revisions
Jorge Stolfi (talk | contribs) m →top: chemspider id |
Jorge Stolfi (talk | contribs) Relation to disilicic acid. Preparation. |
||
| Line 43: | Line 43: | ||
==Reactions== |
==Reactions== |
||
Like other silicic acids, pyrosilicic acid is unstable in solution, and tends to polymerize or hydrolyze.<ref name=goto>Katsumi Goto (1956): "Effect of pH on Polymerization of Silicic Acid". ''Journal of Physical Chemistry'', volume 60, issue 7, pages 1007–1008. {{doi|10.1021/j150541a046}}</ref><ref name=bech>M. F. Bechtold (1955): "Polymerization and Properties of Dilute Aqueous Silicic Acid from Cation Exchange". ''Journal of Physical Chemistry'', volume 59, issue 6, pages 532–541. {{doi|10.1021/j150528a013}}</ref><ref name=ramberg>Hans Ramberg (1952): "Chemical Bonds and Distribution of Cations in Silicates". ''The Journal of Geology'' volume 60, issue 4, pages 331-355. {{doi|10.1086/625982}}</ref> |
Like other silicic acids, pyrosilicic acid is unstable in solution, and tends to polymerize, depolymerize, or hydrolyze.<ref name=goto>Katsumi Goto (1956): "Effect of pH on Polymerization of Silicic Acid". ''Journal of Physical Chemistry'', volume 60, issue 7, pages 1007–1008. {{doi|10.1021/j150541a046}}</ref><ref name=bech>M. F. Bechtold (1955): "Polymerization and Properties of Dilute Aqueous Silicic Acid from Cation Exchange". ''Journal of Physical Chemistry'', volume 59, issue 6, pages 532–541. {{doi|10.1021/j150528a013}}</ref><ref name=ramberg>Hans Ramberg (1952): "Chemical Bonds and Distribution of Cations in Silicates". ''The Journal of Geology'' volume 60, issue 4, pages 331-355. {{doi|10.1086/625982}}</ref> In particular, it can be converted to or from [[disilicic acid]] |
||
: {{chem|H|2|Si|2|O|5}} (disilicic acid) + 2{{chem|H|2|O}} ↔ {{chem|(HO)|3|Si}}–O–{{chem|Si|(OH)|3}} (pyrosilicic acid) |
|||
It can also be [[hydrolized]] to [[monomer]]ic orthosilicic acid: |
|||
: {{chem|(HO)|3|Si}}–O–{{chem|Si|(OH)|3}} (pyrosilicic acid) + {{chem|H|2|O}} ↔ {{chem|(HO)|3|Si}}–OH + HO–{{chem|Si|(OH)|3}} (orthosilicic acid) |
|||
==Preparation== |
|||
Dilute solutions of pyrosilicic acid can be prepared by removing the [[sodium]] cations from solutions of [[sodium pyrosilicate]] with an [[ion exchange resin]].<ref name=gye>W. E. Gye and W. J. Purdy (1922): "The Poisonous Properties of Colloidal Silica. I: The Effects of the Parenteral Administration of Large Doses" ''British Journal of Experimental Pathology'', volume 3, issue 2, pages 75–85. PMCID: PMC2047780</ref> |
|||
Pyrosilicic acid can be prepared also by dissolving a solution of [[sodium pyrosilicate]], which is naturally alkaline, in a solution of [[sulfuric acid]], so as to rapidly achieve an acidic solution with low silica concentration, such as 0.1% {{chem|Si|O|2}} and pH of 2.5. In those conditions, the polymerization and depolymerization reactions above are fairly slow, and the pyrosilicate or disilicate from the original solution is converted to pyrosilicic acid, which persists for 10 minutes or more.<ref name=GBAlex>G. B. Alexander (1953): "The Reaction of Low Molecular Weight Silicic Acids with Molybdic Acid". ''Journal of the American Chemical Society, volume 75, issue 22, pages 5655–5657. {{doi|10.1021/ja01118a054}}</ref> |
|||
Pyrosilicic acid can also be obtained by controlled hydrolysis of the corresponding organic ester, such as [[hexaethyl pyrosilicate]], with dilute sulfuric acid.<ref name=GBAlex/> |
|||
==References== |
==References== |
||
Revision as of 04:32, 28 May 2018
| |
| Names | |
|---|---|
| IUPAC name
Trihydroxysilyl trihydrogen orthosilicate[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H6O7Si2 | |
| Molar mass | 174.211 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
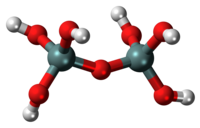
Pyrosilicic acid is the chemical compound with formula H
6Si
2O
7, or (HO)
3Si-O-Si(OH)
3. It is one of the silicic acids. It has never been isolated in pure form, but it is assumed to be present in solutions of silicon dioxide (silica) SiO
2 in water, in equilibrium with other silicic acids and silicate ions.[1]
The salts of pyrosilicic acid are called pyrosilicates, such as sodium pyrosilicate, and are stable. They occur in nature as a class of silicate minerals, specificlaly the sorosilicates.
Reactions
Like other silicic acids, pyrosilicic acid is unstable in solution, and tends to polymerize, depolymerize, or hydrolyze.[2][3][4] In particular, it can be converted to or from disilicic acid
- H
2Si
2O
5 (disilicic acid) + 2H
2O ↔ (HO)
3Si–O–Si(OH)
3 (pyrosilicic acid)
It can also be hydrolized to monomeric orthosilicic acid:
- (HO)
3Si–O–Si(OH)
3 (pyrosilicic acid) + H
2O ↔ (HO)
3Si–OH + HO–Si(OH)
3 (orthosilicic acid)
Preparation
Dilute solutions of pyrosilicic acid can be prepared by removing the sodium cations from solutions of sodium pyrosilicate with an ion exchange resin.[5]
Pyrosilicic acid can be prepared also by dissolving a solution of sodium pyrosilicate, which is naturally alkaline, in a solution of sulfuric acid, so as to rapidly achieve an acidic solution with low silica concentration, such as 0.1% SiO
2 and pH of 2.5. In those conditions, the polymerization and depolymerization reactions above are fairly slow, and the pyrosilicate or disilicate from the original solution is converted to pyrosilicic acid, which persists for 10 minutes or more.[6]
Pyrosilicic acid can also be obtained by controlled hydrolysis of the corresponding organic ester, such as hexaethyl pyrosilicate, with dilute sulfuric acid.[6]
References
- ^ a b "Pyrosilicic acid". Chemspider webpage, CSID:55684, accessed on 2018-05-26
- ^ Katsumi Goto (1956): "Effect of pH on Polymerization of Silicic Acid". Journal of Physical Chemistry, volume 60, issue 7, pages 1007–1008. doi:10.1021/j150541a046
- ^ M. F. Bechtold (1955): "Polymerization and Properties of Dilute Aqueous Silicic Acid from Cation Exchange". Journal of Physical Chemistry, volume 59, issue 6, pages 532–541. doi:10.1021/j150528a013
- ^ Hans Ramberg (1952): "Chemical Bonds and Distribution of Cations in Silicates". The Journal of Geology volume 60, issue 4, pages 331-355. doi:10.1086/625982
- ^ W. E. Gye and W. J. Purdy (1922): "The Poisonous Properties of Colloidal Silica. I: The Effects of the Parenteral Administration of Large Doses" British Journal of Experimental Pathology, volume 3, issue 2, pages 75–85. PMCID: PMC2047780
- ^ a b G. B. Alexander (1953): "The Reaction of Low Molecular Weight Silicic Acids with Molybdic Acid". Journal of the American Chemical Society, volume 75, issue 22, pages 5655–5657. doi:10.1021/ja01118a054
