User:Simoncaulton/sandbox: Difference between revisions
Simoncaulton (talk | contribs) No edit summary |
Simoncaulton (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
[[ |
[[Image:Contact system.svg|400px|thumb|right|The two arms of the contact system. PKa's cleavage of HK liberates BK and promotes inflammation. FXIIa's cleavage of FXI initiates coagulation.]] |
||
The '''contact activation system''' or '''CAS''' involves three proteins: [[factor XII]] (FXII), [[prekallikrein]] (PK) and [[kininogen|high molecular weight kiningogen]] (HK). FXII and PK are [[protease]]s and HK is a non-enzymatic co-factor. The CAS can activate the [[kinin–kallikrein system]] and [[blood coagulation]] through its ability to activate multiple downstream proteins. The CAS is initiated when FXII binds to a surface and reciprocal activation of FXII and PK occurs, forming FXIIa and PKa. FXIIa can initiate the [[coagulation cascade]] by cleaving and activating [[factor XI]] (FXI), which leads to formation of a blood clot. Additionally, the CAS can activate the kinin–kallikrein system. Here, PKa cleaves HK to form cHK, releasing a peptide known as [[bradykinin]] (BK). BK and its derivatives bind to bradykinin receptors [[bradykinin receptor B1|B1]] and [[bradykinin receptor B2|B2]] to mediate [[inflammation]].<ref>{{cite journal |last1=Schmaier |first1=AH |title=Physiologic activities of the contact activation system. |journal=Thrombosis research |date=May 2014 |volume=133 Suppl 1 |pages=S41-4 |doi=10.1016/j.thromres.2014.03.018 |pmid=24759141}}</ref><ref>{{cite journal |last1=de Maat |first1=S |last2=Tersteeg |first2=C |last3=Herczenik |first3=E |last4=Maas |first4=C |title=Tracking down contact activation - from coagulation in vitro to inflammation in vivo. |journal=International journal of laboratory hematology |date=June 2014 |volume=36 |issue=3 |pages=374-81 |doi=10.1111/ijlh.12222 |pmid=24750684}}</ref> |
The '''contact activation system''' or '''CAS''' involves three proteins: [[factor XII]] (FXII), [[prekallikrein]] (PK) and [[kininogen|high molecular weight kiningogen]] (HK). FXII and PK are [[protease]]s and HK is a non-enzymatic co-factor. The CAS can activate the [[kinin–kallikrein system]] and [[blood coagulation]] through its ability to activate multiple downstream proteins. The CAS is initiated when FXII binds to a surface and reciprocal activation of FXII and PK occurs, forming FXIIa and PKa. FXIIa can initiate the [[coagulation cascade]] by cleaving and activating [[factor XI]] (FXI), which leads to formation of a blood clot. Additionally, the CAS can activate the kinin–kallikrein system. Here, PKa cleaves HK to form cHK, releasing a peptide known as [[bradykinin]] (BK). BK and its derivatives bind to bradykinin receptors [[bradykinin receptor B1|B1]] and [[bradykinin receptor B2|B2]] to mediate [[inflammation]].<ref>{{cite journal |last1=Schmaier |first1=AH |title=Physiologic activities of the contact activation system. |journal=Thrombosis research |date=May 2014 |volume=133 Suppl 1 |pages=S41-4 |doi=10.1016/j.thromres.2014.03.018 |pmid=24759141}}</ref><ref>{{cite journal |last1=de Maat |first1=S |last2=Tersteeg |first2=C |last3=Herczenik |first3=E |last4=Maas |first4=C |title=Tracking down contact activation - from coagulation in vitro to inflammation in vivo. |journal=International journal of laboratory hematology |date=June 2014 |volume=36 |issue=3 |pages=374-81 |doi=10.1111/ijlh.12222 |pmid=24750684}}</ref> |
||
==Surfaces== |
==Surfaces and activation== |
||
Artificial negatively charged substances that activate FXII include L-homocysteine, heparan sulfates, chondroitin sulfates, dermatan sulfate, uric acid crystals, lipoproteins, ferritin and porphyrins. However, the physiological substances or surfaces that activate FXII are still under debate. These may include proteins, such as gC1q-R, aggregated proteins, amyloid, collagen, nucleic acids, and polyphosphates.<ref>{{cite journal |last1=Ghebrehiwet |first1=B |last2=Kaplan |first2=AP |last3=Joseph |first3=K |last4=Peerschke |first4=EI |title=The complement and contact activation systems: partnership in pathogenesis beyond angioedema. |journal=Immunological reviews |date=November 2016 |volume=274 |issue=1 |pages=281-289 |doi=10.1111/imr.12469 |pmid=27782339}}</ref><ref>{{cite journal |last1=Naudin |first1=C |last2=Burillo |first2=E |last3=Blankenberg |first3=S |last4=Butler |first4=L |last5=Renné |first5=T |title=Factor XII Contact Activation. |journal=Seminars in thrombosis and hemostasis |date=November 2017 |volume=43 |issue=8 |pages=814-826 |doi=10.1055/s-0036-1598003 |pmid=28346966}}</ref><ref>{{cite journal |last1=Schmaier |first1=AH |title=The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. |journal=Journal of thrombosis and haemostasis : JTH |date=January 2016 |volume=14 |issue=1 |pages=28-39 |doi=10.1111/jth.13194 |pmid=26565070}}</ref> The ability of FXII to bind to negatively charged surfaces and activate coagulation forms the basis of the [[aPTT]] test, in which artificial materials act as a surface for contact activation. This test is used to measure the contact activation pathway (intrinsic pathway) and the common pathway of clotting.<ref>{{cite journal |last1=Naudin |first1=C |last2=Burillo |first2=E |last3=Blankenberg |first3=S |last4=Butler |first4=L |last5=Renné |first5=T |title=Factor XII Contact Activation. |journal=Seminars in thrombosis and hemostasis |date=November 2017 |volume=43 |issue=8 |pages=814-826 |doi=10.1055/s-0036-1598003 |pmid=28346966}}</ref> FXII is a [[zymogen]], which means that it requires processing to attain its catalytic protease activity. Upon binding to surfaces, FXII alters in its conformation, giving it low-level protease activity. This change in conformation also promotes its cleavage by PKa and cleavage by FXIIa itself. FXIIa can cleave PK producing PKa, producing a positive feed-back to activate both enzymes. HK binds to PK and is required to locate PK at the surface for activation by FXII.<ref>{{cite journal |last1=de Maat |first1=S |last2=Tersteeg |first2=C |last3=Herczenik |first3=E |last4=Maas |first4=C |title=Tracking down contact activation - from coagulation in vitro to inflammation in vivo. |journal=International journal of laboratory hematology |date=June 2014 |volume=36 |issue=3 |pages=374-81 |doi=10.1111/ijlh.12222 |pmid=24750684}}</ref> |
|||
The ability of FXII to bind to surfaces and activate coagulation forms the basis of the [[aPTT]] test, in which artificial materials act as a surface for contact activation. This test is used to measure the contact activation pathway (intrinsic pathway) and the common pathway of clotting.<ref>{{cite journal |last1=Naudin |first1=C |last2=Burillo |first2=E |last3=Blankenberg |first3=S |last4=Butler |first4=L |last5=Renné |first5=T |title=Factor XII Contact Activation. |journal=Seminars in thrombosis and hemostasis |date=November 2017 |volume=43 |issue=8 |pages=814-826 |doi=10.1055/s-0036-1598003 |pmid=28346966}}</ref> |
|||
==Phyiological roles== |
==Phyiological roles== |
||
Although the contact system can activate FXI and the subsequent clotting cascade, and it is routinely observed to activate coagulation in the presence of medical devices, the actual role of the contact system in normal physiological coagulation remains contentious. This is primarily due to the fact that deficiencies in the contact system proteins FXII, PK and HK do not produce bleeding disorders. |
Although the contact system can activate FXI and the subsequent clotting cascade, and it is routinely observed to activate coagulation in the presence of medical devices,<ref>{{cite journal |last1=Jaffer |first1=IH |last2=Fredenburgh |first2=JC |last3=Hirsh |first3=J |last4=Weitz |first4=JI |title=Medical device-induced thrombosis: what causes it and how can we prevent it? |journal=Journal of thrombosis and haemostasis : JTH |date=June 2015 |volume=13 Suppl 1 |pages=S72-81 |doi=10.1111/jth.12961 |pmid=26149053}}</ref> the actual role of the contact system in normal physiological coagulation remains contentious. This is primarily due to the fact that deficiencies in the contact system proteins FXII, PK and HK do not produce bleeding disorders.<ref>{{cite journal |last1=de Maat |first1=S |last2=Tersteeg |first2=C |last3=Herczenik |first3=E |last4=Maas |first4=C |title=Tracking down contact activation - from coagulation in vitro to inflammation in vivo. |journal=International journal of laboratory hematology |date=June 2014 |volume=36 |issue=3 |pages=374-81 |doi=10.1111/ijlh.12222 |pmid=24750684}}</ref> |
||
The contact activation system's physiological role in the kinin-kallikrein system is more clear. Here, after activation of PK to PKa by FXIIa, PKa cleaves HK. This produces cleaved HK (cHK), releasing a small peptide known as bradykinin. This peptide binds to bradykinin receptor B2 and its derivative, Des-Arg9-bradykinin binds to bradykinin receptor B1. Upon ligand binding, these receptors mediate inflammatory responses. |
|||
==Roles in disease== |
==Roles in disease== |
||
Revision as of 10:49, 31 July 2018
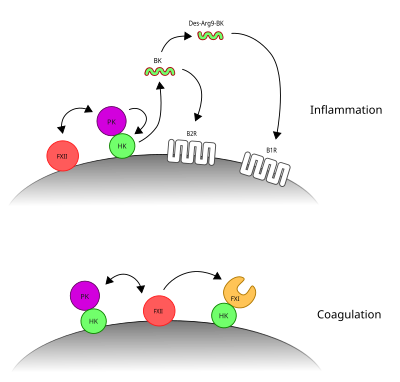
The contact activation system or CAS involves three proteins: factor XII (FXII), prekallikrein (PK) and high molecular weight kiningogen (HK). FXII and PK are proteases and HK is a non-enzymatic co-factor. The CAS can activate the kinin–kallikrein system and blood coagulation through its ability to activate multiple downstream proteins. The CAS is initiated when FXII binds to a surface and reciprocal activation of FXII and PK occurs, forming FXIIa and PKa. FXIIa can initiate the coagulation cascade by cleaving and activating factor XI (FXI), which leads to formation of a blood clot. Additionally, the CAS can activate the kinin–kallikrein system. Here, PKa cleaves HK to form cHK, releasing a peptide known as bradykinin (BK). BK and its derivatives bind to bradykinin receptors B1 and B2 to mediate inflammation.[1][2]
Surfaces and activation
Artificial negatively charged substances that activate FXII include L-homocysteine, heparan sulfates, chondroitin sulfates, dermatan sulfate, uric acid crystals, lipoproteins, ferritin and porphyrins. However, the physiological substances or surfaces that activate FXII are still under debate. These may include proteins, such as gC1q-R, aggregated proteins, amyloid, collagen, nucleic acids, and polyphosphates.[3][4][5] The ability of FXII to bind to negatively charged surfaces and activate coagulation forms the basis of the aPTT test, in which artificial materials act as a surface for contact activation. This test is used to measure the contact activation pathway (intrinsic pathway) and the common pathway of clotting.[6] FXII is a zymogen, which means that it requires processing to attain its catalytic protease activity. Upon binding to surfaces, FXII alters in its conformation, giving it low-level protease activity. This change in conformation also promotes its cleavage by PKa and cleavage by FXIIa itself. FXIIa can cleave PK producing PKa, producing a positive feed-back to activate both enzymes. HK binds to PK and is required to locate PK at the surface for activation by FXII.[7]
Phyiological roles
Although the contact system can activate FXI and the subsequent clotting cascade, and it is routinely observed to activate coagulation in the presence of medical devices,[8] the actual role of the contact system in normal physiological coagulation remains contentious. This is primarily due to the fact that deficiencies in the contact system proteins FXII, PK and HK do not produce bleeding disorders.[9]
The contact activation system's physiological role in the kinin-kallikrein system is more clear. Here, after activation of PK to PKa by FXIIa, PKa cleaves HK. This produces cleaved HK (cHK), releasing a small peptide known as bradykinin. This peptide binds to bradykinin receptor B2 and its derivative, Des-Arg9-bradykinin binds to bradykinin receptor B1. Upon ligand binding, these receptors mediate inflammatory responses.
Roles in disease
Heriditary angioedema [10]
References
- ^ Schmaier, AH (May 2014). "Physiologic activities of the contact activation system". Thrombosis research. 133 Suppl 1: S41-4. doi:10.1016/j.thromres.2014.03.018. PMID 24759141.
- ^ de Maat, S; Tersteeg, C; Herczenik, E; Maas, C (June 2014). "Tracking down contact activation - from coagulation in vitro to inflammation in vivo". International journal of laboratory hematology. 36 (3): 374–81. doi:10.1111/ijlh.12222. PMID 24750684.
- ^ Ghebrehiwet, B; Kaplan, AP; Joseph, K; Peerschke, EI (November 2016). "The complement and contact activation systems: partnership in pathogenesis beyond angioedema". Immunological reviews. 274 (1): 281–289. doi:10.1111/imr.12469. PMID 27782339.
- ^ Naudin, C; Burillo, E; Blankenberg, S; Butler, L; Renné, T (November 2017). "Factor XII Contact Activation". Seminars in thrombosis and hemostasis. 43 (8): 814–826. doi:10.1055/s-0036-1598003. PMID 28346966.
- ^ Schmaier, AH (January 2016). "The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities". Journal of thrombosis and haemostasis : JTH. 14 (1): 28–39. doi:10.1111/jth.13194. PMID 26565070.
- ^ Naudin, C; Burillo, E; Blankenberg, S; Butler, L; Renné, T (November 2017). "Factor XII Contact Activation". Seminars in thrombosis and hemostasis. 43 (8): 814–826. doi:10.1055/s-0036-1598003. PMID 28346966.
- ^ de Maat, S; Tersteeg, C; Herczenik, E; Maas, C (June 2014). "Tracking down contact activation - from coagulation in vitro to inflammation in vivo". International journal of laboratory hematology. 36 (3): 374–81. doi:10.1111/ijlh.12222. PMID 24750684.
- ^ Jaffer, IH; Fredenburgh, JC; Hirsh, J; Weitz, JI (June 2015). "Medical device-induced thrombosis: what causes it and how can we prevent it?". Journal of thrombosis and haemostasis : JTH. 13 Suppl 1: S72-81. doi:10.1111/jth.12961. PMID 26149053.
- ^ de Maat, S; Tersteeg, C; Herczenik, E; Maas, C (June 2014). "Tracking down contact activation - from coagulation in vitro to inflammation in vivo". International journal of laboratory hematology. 36 (3): 374–81. doi:10.1111/ijlh.12222. PMID 24750684.
- ^ De Maat, S; Hofman, ZLM; Maas, C (19 June 2018). "Hereditary angioedema: the plasma contact system out of control". Journal of thrombosis and haemostasis : JTH. doi:10.1111/jth.14209. PMID 29920929.
