Halogenation: Difference between revisions
interwiki (sk) |
he+ |
||
| Line 26: | Line 26: | ||
[[Category:Inorganic reactions]] |
[[Category:Inorganic reactions]] |
||
[[he:הלוגנציה]] |
|||
[[no:Halogenering]] |
[[no:Halogenering]] |
||
[[pt:Halogenação]] |
[[pt:Halogenação]] |
||
Revision as of 12:26, 5 November 2006
Halogenation is a chemical reaction that replaces a hydrogen atom with a halogen atom. More specific descriptions exist that specify the type of halogen: fluorination, chlorination, bromination, and iodination.
In a Markovnikov addition reaction, a halogen like bromine is reacted with an alkene which causes the π-bond to break forming an haloalkane. This makes the hydrocarbon more reactive and bromine as it turns out, is a good leaving group in further chemical reactions such as nucleophilic aliphatic substitution reactions and elimination reactions
Several types of halogenation exist, including:
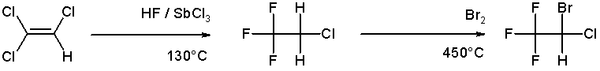
An example of halogenation can be found in the organic synthesis of the anesthetic halothane from trichloroethylene which involves a high temperature bromination in the second step [1]:
See also
- Haloalkane (Alkyl halide)
- Halogenoarene (Aryl halide)
- Haloketone
- Electrophilic substitution
References
- ^ Synthesis of essential drugs, Ruben Vardanyan, Victor Hruby; Elsevier 2005 ISBN 0-444-52166-6
